Diagnosis, management and prevention of thrombotic complications in microsurgical breast reconstruction: a review of the literature
Abstract
Autologous free tissue transfer is a safe and effective option for breast reconstruction. It is an increasingly utilized technique with well-demonstrated improved patient satisfaction and quality of life. Microvascular thrombosis is a rare but significant complication of microsurgical breast reconstruction, often resulting in flap failure. Proper diagnosis and timely management of this complication are essential to free flap salvage. While microvascular thrombosis poses a threat to flap survival, several methods may be employed to mitigate its more devastating effects. Here, we present a comprehensive review of arterial and venous thrombotic complications in both the intraoperative and postoperative settings. We discuss preoperative risk assessment, methods for flap monitoring, and operative and medical management of thrombotic complications. We present an updated algorithm for the intraoperative management of microvascular thrombosis adapted to reflect the most recent literature and our novel algorithm for the postoperative management of microvascular thrombosis.
Keywords
INTRODUCTION
Microsurgical autologous free tissue transfer has become a widely practiced technique for breast reconstruction with improved patient satisfaction and quality of life[1-3]. With advances in flap monitoring techniques and medical and surgical management, autologous free tissue transfer is now a safe and effective procedure with high success rates[4,5]. While uncommon, microvascular thrombosis remains a serious complication, occurring in 1.5%-6.2% of breast reconstruction cases, with up to 75% of those cases ultimately resulting in flap failure[6,7]. In this review, we present an overview of the risk factors associated with microvascular thrombosis in free tissue transfer as well as its diagnosis and treatment to facilitate a comprehensive understanding of this potentially devastating complication.
PREOPERATIVE CONSIDERATIONS
Risk factors
Risk factors for flap thrombosis can be categorized by their association with one of the three components of Virchow's triad: stasis, endothelial injury, and hypercoagulability. While flap thrombosis is usually attributed to suboptimal intraoperative technique and flap monitoring, acquired or inherited factors that influence the coagulation cascade must be accounted for during patient selection and preoperative optimization. Preoperative consultation should therefore always pay close attention to family and prior medical history suggestive of coagulopathy, potential secondary causes of bleeding disorders, and medications.
While the impact of patient factors on venous thromboembolism has been well studied, data on microvascular thrombosis rates in breast reconstruction are less robust and often discordant. Many studies are limited by small samples and event numbers, inclusion of a single institution, heterogeneity of reconstruction technique, or insufficient controlling of confounding variables[8]. In this section, we summarize the current literature available for commonly encountered patient factors that are often thought to be associated with flap thrombosis, and review management strategies for each.
Hereditary thrombophilia
While there have been individual case reports of thrombosis with flap loss in Factor V Leiden patients undergoing microsurgical breast reconstruction, they cannot be used to accurately estimate thrombotic risk[9-11]. In a retrospective study of 2032 consecutive free flaps (not limited to breast reconstruction), 58 of which were performed on patients with prior macrovascular thrombosis and/or known thrombophilia, Wang et al. found significantly higher rates of flap thrombosis and flap failure among the hypercoagulable group[12]. However, Pannucci et al. noted that this study failed to recognize that flap thrombosis occurred only among hypercoagulable patients with prior history of macrovascular thrombosis or another acquired hypercoagulable disorder[13]. Flap thrombosis did not occur in patients who had known hereditary thrombophilia without any additional history, suggesting that hereditary thrombophilias are less predictive of flap outcomes than acquired thrombophilias or prior history of thrombosis.
Based on these findings, Pannucci et al. recommend preoperative screening according to personal and family history of thrombosis, acquired risk factors, and Caprini score; if the patient has elevated risk determined by the screening, they should be referred to a hematologist[13]. This approach deviates from the algorithm previously proposed by Friedman et al., who suggest that surgeons should order thrombophilia testing if there is a concern for thrombosis risk and refer to hematology only if the testing is positive[14]. Pannucci et al. argue that decisions on thrombophilia testing should be deferred to the hematologist, because there is no evidence supporting hereditary thrombophilia as a risk factor for flap thrombosis[13]. At our institution, all patients with a history of VTE or hereditary thrombophilia are routinely evaluated by Hematology for risk optimization and operative clearance. Typically, a prophylactic regimen of either an injectable low molecular weight heparin (LMWH) or an oral, direct factor Xa inhibitor is recommended for 1-4 weeks postoperatively. All patients are placed on heparin prophylaxis intraoperatively.
Obesity
Obesity in patients undergoing microsurgical breast reconstruction has been associated with increased risks of partial flap necrosis, fat necrosis, and venous congestion[15-17]. In a study of 936 free transverse rectus abdominis muscle (TRAM) flap cases, Chang et al. found that obese and overweight patients had a significantly higher overall flap complication rate of 39.1% (compared to 20.4% among normal-weight patients), which included a total flap loss, hematoma, seroma, and skin necrosis[18]. Notably, they did not find any difference in the rate of vessel thrombosis. Hanwright et al. found similar results in their analysis of free flap breast reconstruction cases taken from the American College of Surgeons National Surgical Quality Improvement Program (ACS-NSQIP) database[17]. When classified into class I (BMI of 30 to < 35), class II (BMI of 35 to < 40) and class III (BMI ≤ 40) obesity, Fischer et al. found that class III obesity patients had significantly higher rates of flap loss and trended toward higher rates of intraoperative arterial thrombosis[19]. Similarly, Schaverien et al. found that class III obesity was associated with a significantly higher risk of complete flap failure, donor-site complications, and overall complications[20].
Given the increased risk of complications associated especially with morbid obesity, careful patient selection is necessary and patients with class III obesity may be advised to reduce their weight prior to surgery[19]. While no studies demonstrate a specific BMI that provides an acceptable risk to proceed with surgery, statistically significant differences in complications tend to increase proportionally as BMI increases[21]. In our practice, we do not use a specific BMI cutoff to assess surgical candidacy, but we candidly discuss the increased risks of partial or total flap failure with all class III patients seeking microvascular breast reconstruction.
Tobacco use
Despite experimental evidence on the detrimental effects of tobacco smoke exposure on thrombogenicity, clinical studies on free flap transfers in breast reconstruction have demonstrated conflicting results, with the majority suggesting a less significant effect[22-24]. Khouri et al. found that there was no significant effect of tobacco use on flap outcome[25]. Masoomi et al. and Arnez et al. found no significance in flap loss or vascular thrombosis rates in smokers compared to non-smokers[7,26]. Despite studies showing a weak association between flap thrombosis and smoking, patients should still be advised to cease smoking a minimum of 3 weeks prior to surgery, a widely advocated practice due to the established risk of poor wound healing[27].
Radiation
Patients seeking breast reconstruction following post-mastectomy radiation therapy have become increasingly common. Although radiotherapy is known to impair wound healing, its effect on microanastomoses remains an area of ongoing study[28]. Findings from animal studies on irradiated microanastomoses have been variable, with some demonstrating significant change in patency due to intimal hyperplasia as well as increased thrombosis risk, and others showing no such effects[29-32]. Fracol et al. and Fosnot et al. found a significantly higher risk of any intraoperative vascular complication in radiated fields compared to non-radiated fields, but no significant differences in arterial or venous thrombosis rates both intra- and postoperatively, and no overall difference in the rate of flap loss associated with radiation[33,34].
Complication rates have been shown to be lower in patients who delay breast reconstruction until after radiation is complete[35,36]. Though there is little consensus in the literature regarding the optimal timing of autologous reconstruction following radiation therapy, the majority of surgeons report waiting for 4-6 or 7-12 months after the end of radiation, with patient preference and desire to optimize aesthetic outcomes being the primary drivers of the timing selected[37,38]. Notably, Baumann et al. found that among patients receiving delayed abdominal free flap breast reconstruction, flap loss and reoperation rate was higher following reconstruction within 12 months of completion of radiation therapy[39]. At our institution, we routinely delay breast reconstruction for at least 6-12 months after the last radiation treatment, depending on the total radiation dosage, the patient’s symptoms, and the effects noted on physical examination.
Hormonal therapy
Anti-estrogen therapies used in the adjuvant treatment of hormone-sensitive breast cancers, most notably tamoxifen, have been shown to be associated with venous thromboembolism[40-42]. However, results from studies examining the effect of hormone therapy on flap thrombosis are conflicting[43-47]. Although there are studies suggesting discontinuation of hormone therapy 2 to 4 weeks prior to breast reconstruction, there is no consensus in the literature on whether cessation is necessary[44,46-50]. Until a more definitive conclusion is reached, at our institution, we typically recommend holding hormone therapy for a period of 2 weeks before and after surgery, given the low oncologic risk of short-term cessation.
Thromboprophylaxis
Administering antithrombotic agents as a prophylactic measure against microvascular thrombosis is a common but non-standardized practice. Protocols for thromboprophylaxis are based largely on individual surgeon preference and opinion, and thus vary widely with regard to agents, dosage, schedule, and duration. In this section, we describe commonly used antithrombotic agents and present an overview of recent evidence on thromboprophylaxis protocols as well as our own institution’s regimen.
Heparin
Heparin binds and enhances the activity of antithrombin III, which in turn inhibits the coagulation cascade and effectively blocks clot formation and growth. Although animal studies have demonstrated improvement in microvascular thrombosis rates with heparin, clinical findings have been conflicting[51,52]. Lighthall et al. and Zhou et al. found no significant differences in flap failure rates between patients with postoperative heparin and patients with no postoperative anticoagulants[53,54]. Multiple studies have also found no significant differences in microvascular thrombosis rates between cases performed with and without intraoperative heparin[25,55,56]. However, an earlier study by Kroll et al. found that free flap patients dosed with postoperative heparin had lower pedicle thrombosis rates and a trend toward lower flap loss compared to patients with no postoperative anticoagulant agents[56].
Aspirin
Aspirin inhibits the production of thromboxane A2 by platelets, which prevents further platelet activation and aggregation. Similar to heparin, the effectiveness of aspirin for flap thrombosis prevention is unclear despite its widespread use. When used alone, aspirin has not been found to be effective for thromboprophylaxis and may be associated with higher complication rates[53]. Interestingly, Ashjian et al. found in a retrospective review of 505 microvascular free flap patients that rates of microvascular thrombosis and flap loss were equivalent between patients who received a postoperative 5-day daily regimen of 325 mg of aspirin and patients who received 5,000 units of LMWH until ambulating[57].
Combination Therapy
Although many recommendations for thromboprophylaxis have been proposed, there is no consensus on any single regimen. Based on experience and literature review, Conrad et al. proposed a protocol consisting of aspirin dosed 1.4 mg/kg/day administered pre- and postoperatively for 2 weeks, with intraoperative heparin as a bolus and local topical agent[58]. Stephan et al. and Brinkman et al. do not recommend aspirin and instead adhere to heparin monotherapy[59,60]. Overall, current evidence seems to suggest that a more conservative approach to prophylactic antithrombotics is warranted. In a recent systematic review, Liu et al. concluded that postoperative antithrombotics including aspirin, dextran, and heparin had no significant effects on flap thrombosis or flap failure, and may increase the risk for hematoma regardless of regimen[61].
At our institution, patients undergoing microsurgical breast reconstruction receive a bolus of heparin 5,000 units subcutaneously, or enoxaparin (40 mg or 0.5 mg/kg if BMI exceeds 40 kg/m2) subcutaneously intraoperatively. Postoperatively, patients receive heparin 5000 units subcutaneously every 8 h, or enoxaparin (same dosing scheme as previously stated) subcutaneously and aspirin 121.5 mg (half a baby aspirin) once per day. In patients deemed at high risk for microvascular thrombosis, enoxaparin is continued for 3-4 weeks postoperatively.
DIAGNOSIS
Intraoperative
Intraoperative assessment of anastomotic patency and detection of microvascular thrombosis allows for rapid surgical correction and is imperative for flap survival. Historically, microsurgeons have relied on clinical judgment and patency testing. This includes visual inspection of the flap for bleeding at the flap edges, acoustic sonography over perforators, and examination of the vessel for visible or palpable pulsations distal to the anastomosis. Patency can further be assessed with the Flicker test and Milking test[62].
If questions regarding flow remain after a simple inspection of the pedicle, more advanced techniques can be used[63-65]. Fluorescent Indocyanine Green (ICG) angiography has since been shown to be a reliable, sensitive, and ultimately cost-effective method for evaluating flap perfusion[64-66]. Specifically, the arterial uptake phase in ICG angiography is highly sensitive and has been well-studied in the detection of arterial thrombosis[67]. The venous phase, and the data on its sensitivity, are less clear, and its interpretation is oftentimes influenced by the experience of the surgeon[68]. Yoshimatsu et al. report success using ICG angiography to detect venous congestion and Sharaf et al. subsequently describe a “pathognomonic heterogeneous or splotchy appearance” within the zone of ICG appearance that is characteristic of venous congestion [Figure 1][69,70].
Figure 1. ICG angiography of DIEP flaps. (A) Heterogenous ICG appearance consistent with compromised venous outflow; (B) Homogenous ICG appearance consistent with healthy venous outflow; This figure is quoted from Sharaf et al. published in Microsurgery by Wiley Periodicals, Inc., copyright 2021[70]. Reprinted with permission from John Wiley and Sons.
Flaps can also be interrogated intraoperatively using advanced flap monitoring techniques typically reserved for the postoperative setting-including Near-Infrared Spectroscopy (NIRS) tissue oximetry (eg., ViOptix) or technologies such as FLIR (Forward Looking Infrared) thermal imaging.
Postoperative
Flap monitoring techniques
It is well established in the literature that early detection of and intervention for microvascular thrombosis maximizes the chance of flap salvage[71,72]. Therefore, having a reliable means of flap monitoring is critical. In 1975, Creech and Miller described the ideal flap monitoring technique as one that does not cause harm to the patient or flap and is rapid, accurate, reliable, cost-effective, and applicable to all flap types[73]. Jones further proposed that the ideal monitor be objective, simple to use for inexperienced personnel, and capable of continuous and prolonged monitoring[74]. While no one flap monitoring technique embodies all of these qualities, multiple technologies have since emerged, often used in combination with conventional techniques.
Physical examination
Physical examination is a commonly practiced method for assessing flap viability. The physical examination should include an assessment of flap color, temperature, turgor, and capillary refill[74,75]. Flap temperature has previously been assessed via touch, temperature probe, temperature-sensitive tape, or handheld contactless thermometer. However, surface temperature monitoring has not been routinely recommended in larger perforator flaps due to its inability to detect changes prior to flap failure or predict reoperation in DIEP flap breast reconstructions[76]. Bleeding is also an important component of the physical examination in flap monitoring. This can be ascertained via needle prick or skin incision; however, both of these techniques can result in transient ecchymosis that may affect accurate flap assessment[74,76].
The physical examination can aid in determining the etiology of flap thrombosis. An arterial thrombosis can be characterized by a cool and pale appearing skin paddle, diminished turgor, and delayed capillary refill. On the contrary, venous thrombosis is often characterized by an edematous and mottled appearing skin paddle with brisk (< 1-2 seconds) capillary refill, increased turgor, and bleeding on needle prick[75].
Vascular flow
Physical examination is typically accompanied by a more objective assessment of vascular flow. Currently, the most widely used techniques include those that monitor vascular flow and those that monitor tissue ischemia [Table 1]. The most commonly used objective assessment is intermittent interrogation of blood flow with an acoustic Doppler. While handheld acoustic Doppler sonography is not capable of performing continuous monitoring, it is a widely available, cost-efficient, and non-invasive method for monitoring vascular flow that can be easily operated by house staff[77].
Intra- and postoperative monitoring techniques for the detection of thrombotic complications in microsurgical breast reconstruction
| Flap Monitoring Technique | Mechanism of Monitoring | Receiver Operating Characteristics | Advantages | Disadvantages | Recommendation for Use |
| Acoustic Doppler Sonography[77] | The location of the arterial and venous anastomoses is marked on the surface of the flap intraoperatively. A Doppler probe is placed on the surface of the skin paddle overlying the vessel. An auditory pulsatile or continuous hum sound confirms arterial or venous patency, respectively | Sensitivity: 50% Specificity: 100% PPV: 100% NPV: 98.6% Accuracy: 98.6% SR: 100% | Non-invasive, readily available, able to distinguish between venous and arterial flow, ease of operator use, and relatively inexpensive. | Unable to perform continuous monitoring, difficult to determine the source of Doppler signal (recipient vs pedicle), no quantitative output, and interpretation dependent on clinical experience | Recommended for routine postoperative monitoring, ideally in conjunction with other continuous/advanced monitoring techniques when available |
| Cook-Swartz Implantable Doppler[162-168] | An electrode mounted on a silicone cuff is secured around the vascular pedicle with a thin wire connecting it to an external monitor. Auditory output is similar to that of acoustic Doppler sonography | Sensitivity: 100% Specificity: 88-100% PPV: 33.3-100% NPV: 100% Accuracy: 88.7-100% SR: 80-100% | Capable of continuous monitoring, able to distinguish between arterial and venous flow, and ease of operator use | Relatively more invasive, no consensus on probe placement, no quantitative output, high false-positive rate, risk of anastomotic rupture when pulling the probe, and risk of thrombosis or vessel kinking from the probe/wire | Not recommended if there is a skin paddle, given the preference for non-invasive modalities. Recommend use in buried flaps |
| Flow Coupler Implantable Doppler[162,169] | A venous coupler is fitted with a Doppler probe with a thin wire connecting it to an external monitor. Auditory output is similar to that of acoustic Doppler sonography | Sensitivity: 100% Specificity: 94-98.1% PPV: 60-66.7% NPV: 100% Accuracy: 94.7-98.2% SR: 75-100% | Easier to place with reduced operative time compared to implantable Doppler alone, capable of continuous monitoring, and ease of operator use | Relatively more invasive, no quantitative output, monitors venous flow only, and risk of thrombosis or vessel kinking from the probe/wire | Not recommended if there is a skin paddle, given the preference for non-invasive modalities. Recommend use in buried flaps |
| Color Duplex Ultrasonography[170,171] | An ultrasound probe and viewing monitor allows direct visualization of vessel patency as well as blood flow velocity and direction | Receiver operating characteristics for the detection of microvascular thrombosis have not been reported in the context of microsurgical breast reconstruction; however, Jacob et al. and Arya et al. have described its potential use | Non-invasive, readily available, provides real-time imaging of anastomotic patency, and provides quantitative output | Unable to perform continuous monitoring, typically requires a radiology technician to perform and a radiologist to interpret, and no comparative studies available on its use in breast flaps, costly | Can consider adjunctive use in the intraoperative and postoperative setting or in buried flaps. However, should not be used as a primary postoperative monitoring tool due to lack of data and operator dependence |
| Laser Doppler Flowmetry[172] | A probe attached to the surface of the skin paddle emits laser light which is reflected back by the movement of red blood cells to calculate their velocity | Sensitivity: 100% Specificity: 100% PPV: 100% NPV: 100% Accuracy: 100% SR: 80% | Non-invasive, capable of continuous monitoring | Monitors at the capillary level only so unable to distinguish between venous and arterial flow, subject to error due to patient movement, no standard criteria for detecting vascular compromise, operator dependent | Not yet advanced enough to be recommended in routine clinical practice |
| Near-Infrared and Visible Light Spectroscopy[77,81-83,172-179] | A probe attached to the surface of the skin paddle emits near-infrared or visible light, which is absorbed by chromophores (oxygenated and deoxygenated hemoglobin). The reduction in light intensity is measured to determine tissue oxygen saturation | Sensitivity: 96.5-100% Specificity: 96.4-100% PPV: 50-100% NPV: 99.8-100% Accuracy: 97-100% SR: 66.7-100% | Non-invasive, capable of continuous monitoring, not sensitive to patient movement, provides quantitative output, criteria for detecting vascular compromise defined, and ease of operator use. | Monitors at the capillary level only so unable to distinguish between venous and arterial flow, potential influence of clinical (ex flap type or skin pigment) and environmental (ambient light) variables, and relatively more costly than Doppler devices | Recommended for continuous postoperative monitoring in conjunction with routine acoustic Doppler sonography and clinical assessment if institutional resources allow |
| Microdialysis[164-168] | A double-lumen microdialysis catheter is introduced into the flap and perfusion fluid is collected. Fluid is subsequently analyzed for products of anaerobic respiration, including low glucose and elevated lactate concentrations | Sensitivity: 100% Specificity: 92.5-100% PPV: 66.7-100% NPV: 100% Accuracy: 93.5-100% SR: 83-100% | Sensitive to flow compromise before clinically apparent, able to monitor buried flaps | Invasive, unable to perform continuous monitoring, sample analysis is not immediate, high false positive rate resulting in unnecessary re-explorations and higher treatment costs, unable to distinguish between venous and arterial flow, and costly | Not yet recommended in routine postoperative breast monitoring, given the presence of other continuous and non-invasive modalities. Can consider use in buried flaps when other forms of monitoring are not feasible |
| Fluorescent ICG Angiography[180,181] | ICG is injected intravenously and fluoresces near-infrared light. An infrared-sensitive camera captures these emissions to provide vessel imaging | Sensitivity: 100% Specificity: 86-100% PPV: 100% NPV 100% Accuracy: 100% SR: 100% | Non-invasive, provides real-time imaging of anastomotic patency, and highly sensitive to arterial thrombosis | Unable to perform continuous monitoring, not readily available at the bedside, less sensitive to venous thrombosis, costly | Recommended for intraoperative monitoring but not as a primary monitoring tool postoperatively |
Additional Doppler technologies have since been developed to allow for continuous flap monitoring, including the Cook-Swartz implantable Doppler, flow coupler implantable Doppler, and laser Doppler flowmetry[78]. Given the invasive nature of implantable Doppler, some authors suggest that vessel compression and anastomotic injury by the implanted cuff or wire should be considered[79]. In addition, laser Doppler is a promising non-invasive option but lacks consensus values for detection and thus remains experimental[80].
At our institution, we recommend regularly spaced intervals of acoustic Doppler sonography, ideally in combination with continuous tissue oximetry-based monitoring. However, implantable Doppler is used for continuous monitoring when a skin paddle is not available, such as in buried skin flaps or muscle flaps without a skin paddle.
Tissue ischemia
Near-Infrared Spectroscopy (NIRS) tissue oximetry is an important tool that has been shown to detect flap compromise before it is clinically apparent, decrease rates of flap loss, and improve rates of flap salvage compared to conventional techniques[81]. While more expensive upfront than continuous Doppler techniques, NIRS has demonstrated an overall potential cost benefit across multiple studies[75,82-84]. Pelletier et al. found an average reduction of $1,937 per patient when monitored on the surgical floor with NIRS tissue oximetry compared to the surgical intensive care unit (ICU)[82]. Additionally, given the quantitative output of NIRS compared to Doppler technology, an automated text message alert system has been developed, allowing for rapid notification of the surgical team[85]. The potential for decreased time to re-exploration, a critical factor in flap salvage, makes NIRS a promising technology. While NIRS is a valuable tool to continuously monitor flaps with a skin paddle, no single monitoring device should supersede a thorough physical examination and individual clinical experience.
Flap monitoring protocols
Currently, there is no universally recognized protocol or standardized practice for flap monitoring following microsurgical breast reconstruction. Historically, flaps have been monitored in an intensive care or step-down setting for 1 or more days postoperatively, given that the majority of complications occur within the first 24-48 h after surgery[85,86]. With advancements in flap monitoring technologies, many institutions have altered their protocols to allow for early disposition to the floor without increasing the risk of flap failure or postoperative complications[82,83,87-89]. In line with the available literature, we present our institution's flap monitoring protocol in [Figure 2], adapted from Khansa et al. to reflect our institution’s recommendation for timing and location of monitoring, and criteria for takeback[90]. Nonetheless, we recognize that ultimately a surgeon’s approach to flap monitoring should take into account individual patient factors, institutional resources, and the evolving literature.
Figure 2. Algorithm for postoperative flap monitoring. This figure is adapted from Khansa et al. published in Microsurgery by Wiley Periodicals, Inc., copyright 2013[90]. Adapted with permission from John Wiley and Sons.
MANAGEMENT
Arterial insufficiency
Intraoperative management of arterial insufficiency
Upon intraoperative detection of signs of arterial insufficiency, the anastomosis should promptly be re-examined. The vessel should be inspected for extrinsic compression, vessel spasm, and positional issues such as kinking[91]. The anastomosis should be assessed for the presence or absence of flow with clinical examinations such as the milking test and acoustic Doppler sonography. If arterial thrombosis is suspected, one or more salvage modalities may be attempted[90]. A detailed algorithm for our approach to the intraoperative management of arterial insufficiency is available in [Figure 3A], adapted from Khansa et al. to reflect our institution’s use of papaverine[90].
Figure 3. Algorithm for intraoperative management of free flap vessel insufficiency. (A) arterial insufficiency; (B) venous insufficiency; This figure is adapted from Khansa et al. published in Microsurgery by Wiley Periodicals, Inc., copyright 2013[90]. Adapted with permission from JohnWiley and Sons.
Arterial flow present
Upon exploration of the pedicle, should arterial Doppler flow be present, a careful clinical examination of the flap and the entire pedicle should be performed. The pedicle should be inspected for any areas that may be prone to twisting, kinking, or external compression. The use of fat grafting over the pedicle can help to maintain the optimal vessel lie. Should the flap appears clinically improved - including the presence of normal capillary refill, turgor, and dermal edge bleeding - it may be carefully re-inset. ICG angiography could be considered to evaluate the flap after inset to ensure the adequacy of flow. If flow is confirmed, close clinical observation in the postoperative period is recommended[90].
Arterial flow diminished
If flow is present but diminished, etiologies can include partial microvascular thrombosis, vasospasm, or suboptimal vessel positioning. Vasospasm is best treated through the avoidance of peripheral vasopressors and the local application of topical vasodilators[92-95]. Topical treatments include a wide variety of vasodilators, including alpha antagonists (eg., phentolamine), calcium channel blockers (eg., nicardipine), direct vasodilators (eg., hydralazine), local anesthetics (eg., lidocaine), and phosphodiesterase inhibitors (eg., papaverine)[96]. As multiple vasodilators have been proven to be efficacious, the precise type of vasodilator and the dosing used is more often based on surgeon experience and availability[95-98]. At our institution, vasospasm is often treated with an adventitial injection of papaverine and warm heparinized saline. If there is a specific point of vasospasm identified, these injections can be combined with careful milking of the pedicle using microforceps or the surgeon’s pinched fingers, a technique that may provide sufficient intraluminal pressure to break the spasm. Should arterial flow not improve after treatment for vasospasm, the anastomosis should be re-explored, as described in the Arterial Flow Absent section.
Arterial flow absent
Time to re-exploration and anastomotic revision is critical to flap survival. If the arterial flow is absent or does not respond to treatment for vasospasm, the anastomosis should be opened and promptly explored. If a thrombus is identified upon opening the arterial anastomosis, heparin irrigation and mechanical thrombectomy or chemical thrombolysis may be necessary in addition to revision of the anastomosis. In cases of arterial thrombosis, heparinized saline is used liberally in a 100 I.U./mL concentration to flush the flap and the anastomosis. A review by Couteau et al. supports this practice, with 9 of 11 animal studies showing improved free flap survival rates with the use of intraoperative heparin irrigation compared to saline[99].
The simplest form of mechanical thrombectomy is the direct removal of the thrombus with standard microforceps. If the thrombosis is detected prior to propagation, direct removal at the proximal end of the anastomosis can be sufficient. However, if thrombus is suspected to be in the distal pedicle, Fogarty catheter-assisted thrombectomy may be necessary[100]. We typically use a Fogarty catheter with a 1- or 2-mm balloon. The catheter may be introduced via the proximal lumen or a distal side branch if one of sufficient size is available for cannulation. The catheter should be carefully passed until it reaches the perforating vessels entering the flap to ensure that the entire vessel is cleared. Prior to withdrawal, the balloon is typically inflated to half its total capacity to minimize damage to the perforator. Multiple passes may be needed to completely eliminate the propagated thrombus[101]. Once the mechanical thrombectomy is complete, heparinized saline flushes can be used to assess the flap’s resistance to flow. If the pedicle can be flushed easily and venous return of the saline is confirmed, a revision of the anastomosis should be attempted. Nevertheless, if resistance to flow is detected, chemical thrombolysis may be needed. At our center, a catheter clearing dose of 1-2 mg of tissue plasminogen activator (tPA) is used. To avoid systemic thrombolysis, we ensure that the flap is isolated from the systemic circulation during the injection of the tPA. After the thrombolytic is allowed to dwell within the flap for several minutes, the flap is again flushed with 300-500 milliliters of heparinized saline to minimize the introduction of systemic tPA after reanastomosis. Furthermore, should an arterial thrombus not be identified after opening the anastomosis, the venous anastomosis should be further explored per the Intraoperative Management of Venous Insufficiency guidelines.
Although the above algorithm is used for complete loss of inflow, it is also possible to have partial loss of arterial flow. If the clinical examination or ICG angiography demonstrates inadequate flow to only a portion of the flap, the presence of partial flap thrombosis should be considered. Partial flap thrombosis, especially if it is thought to be intra-flap thrombosis, is often treated with medical management. At our center, we typically attempt to treat partial intra-flap thrombosis with a combination of chemical thrombolysis (e.g., tPA), anticoagulation and/or simple debridement of the thrombosed portion of the flap.
Postoperative management of arterial insufficiency
Reoperation
If arterial thrombosis is suspected in the postoperative period, expeditious return to the operating room to expose the anastomosis is the most appropriate next step[90]. Time to reoperation is consistently shown to be associated with salvage rates after arterial and venous thrombosis[71,72,102]. Likely secondary to delays in management, the rate of flap salvage in postoperative compromise is less than that of intraoperative compromise[90]. It has been shown that the use of careful continuous postoperative monitoring is associated with a decreased time to diagnosis and operative management of flap thrombosis, and thus an increase in the rate of salvage[81]. The approach to anastomotic assessment and revision is discussed in the intraoperative management section above. Our novel algorithm for the approach to postoperative management of thrombotic complications is available in [Figure 4].
Systemic anticoagulation
In the setting of postoperative arterial thrombosis, the use of systemic anticoagulation varies by surgeon and institution. Many authors report urgent administration of a 5,000-unit bolus of intravenous heparin at the time of reoperation[71,103]. Others report the use of weight-based dosing to achieve an institution-determined therapeutic PTT level[58,103]. Given higher rates of hematoma with systemic heparin use in free tissue transfer, we typically recommend weight-based dosing of intravenous heparin without the use of a bolus[87]. Once the risk of postoperative hemorrhage is deemed sufficiently low, the patient may be transitioned to a low molecular weight heparin injection (eg., enoxaparin). The duration of anticoagulation after flap thrombosis is often driven by surgeon experience. Although there is no definitive data on the optimal duration of therapeutic anticoagulation in this cohort, Khansa et al maintain systemic anticoagulation for at least 4-7 days after reoperation, which is consistent with what other authors report[58,71,90].
Venous insufficiency
Venous congestion and venous thrombosis
The development of venous congestion can be attributed to several etiologies, the most common of which is venous thrombosis[104]. Mechanical factors such as unfavorable flap position, or kinking and compression of the vascular pedicle are common causes of venous insufficiency. Venous thrombosis can occur secondary to a hypercoagulable state, a technical error at the anastomosis, or from prolonged venous congestion or insufficiency from one of the above mechanical factors[105,106]. Finally, venous insufficiency can result from anatomic variability within the flap, especially if the flap contains portions of two perforasomes. Anatomical studies of DIEP and TRAM flaps have shown that normal venous drainage of the lower anterior abdominal subcutaneous tissue and skin occurs primarily through the superficial inferior epigastric vein (SIEV), which is connected to the deep inferior epigastric vein (DIEV) by choke vessels composed of the vena comitantes of the perforators of the deep inferior epigastric artery (DIEA)[107]. Although the majority of DIEP flaps may survive based on the outflow from the DIEV system alone, the venous outflow of some DIEP flaps may be superficially dominant. In these cases, both the DIEV and the SIEV may require anastomosis for adequate venous drainage[107].
Preoperative imaging with CT angiogram, especially among patients with prior surgery in the region of planned flap harvest, may be beneficial in perforator selection and evaluation of venous anatomy. The presence of the SIEV and its caliber should also be evaluated radiologically and intraoperatively. If the SIEV is noted to be of good caliber (> 1.5 mm), it is prudent to preserve adequate length on this vessel to allow for anastomosis.
Intraoperative management of venous insufficiency
As described by Heller and Levin, obstruction of venous outflow can lead to red blood cell extravasation, endothelial breakdown, microcirculation thrombosis, and flap death[108]. Signs of venous congestion in the flap may include rapid capillary refill on the skin paddle (< 1-2 seconds), more profuse dermal bleeding of a darker color, loss of venous Doppler signals in the pedicle and perforators, greater flap turgor, and enlarged secondary veins such as the SIEV. Our algorithm for intraoperative management of suspected venous insufficiency is available in [Figure 3B], adapted from Khansa et al. to reflect our institution’s use of leeching and de-epithelialization[90].
Suspicion for venous congestion requires release of insetting sutures and diligent assessment of vessel position, flap and pedicle orientation within the breast pocket, and presence of hematoma and/or edema requiring evacuation. This should be paired with the assessment of anastomotic flow using Doppler sonography. Secondary veins, such as the SIEV in the case of a DIEP flap, should be examined closely. These veins should be opened to allow for assessment of their outflow. If the secondary veins are noted to have robust outflow, they should be used for venous supercharging of the flap as described below (Venous Flow Present).
Flaps or pedicles can be repositioned to achieve a more favorable lie without kinking. Autologous fat grafts may be used to cushion the pedicle or maintain its position without twists or kinks. Re-evaluation of the venous anastomosis or coupler is critical. A milking test can be used to ensure flow across the anastomosis and throughout the length of the pedicle. If the flap vein was twisted or kinked relative to the recipient vein during anastomosis, the anastomosis may need revision to avoid propagation. Venous vasospasm can cause global congestion, which can usually be resolved by irrigating the vessels with vasodilators (papaverine, lidocaine, verapamil, and nitroglycerin mixture) and warm salinex[109].
Venous flow diminished or absent
If there is absent or diminished flow in the anastomosis after mechanical factors and simple vasospasm have been ruled out, the anastomosis should be taken down and inspected for thrombi. As venous supercharging may be necessary, secondary veins should be examined closely as described below. The artery should also be carefully inspected for signs of diminished flow. If the artery is noted to have abnormal flow, this vessel should be treated using the algorithm above (See intraoperative management of arterial thrombosis). If thrombus is noted in the proximal vein, direct thrombectomy with microforceps should be attempted. If the thrombus is too extensive or distant for this approach, a 1-3 mm Fogarty catheter can be used to attempt thrombus removal in the distal pedicle. Although there is concern that the use of Fogarty catheters on microvasculature can increase the risk of endothelial denudation and thrombogenesis, studies on complication rates following Fogarty catheterization so far have been conflicting and limited by small study samples. While some studies have reported successful flap salvage using the Fogarty catheter, others have found a higher rate of failure in flaps undergoing Fogarty catheter thrombectomy[101,110]. However, as there have been no large-scale studies on the use of Fogarty catheters in flap salvage, and all previous studies are subject to significant selection bias, we believe that Fogarty catheters have an important role in the armamentarium of the reconstructive microsurgeon - especially when attempting to salvage flaps with more extensive or proximal thrombi that are not accessible for direct thrombectomy. After the Fogarty catheter is used, the vessels are typically flushed copiously with heparinized saline as per our arterial algorithm.
If no thrombus is visualized upon taking down the anastomosis, it is possible that there is evidence of intra-flap venous insufficiency or thrombosis. Although ICG angiography is typically used to examine inflow to the flap, venous outflow can be assessed using a washout phase. After the arterial flow is confirmed using the ICG, a second examination can be done after a 2-3 min delay. If ICG dye remains in portions of the flap after this delay, it is likely that these portions may be experiencing venous insufficiency. In cases of intra-flap thrombosis, whether arterial or venous, chemical thrombolysis may be needed (see the arterial treatment algorithm above). If only a portion of the flap is determined to suffer from venous insufficiency, this non-viable tissue may simply be debrided.
Venous flow present
Evidence of persistent flap congestion in the setting of venous patency indicates the need for venous supercharging, or additional venous flow augmentation. Of the two most common autologous breast reconstructions, DIEP flaps are more likely than free TRAM flaps to be complicated by venous congestion requiring flow augmentation, likely due to DIEP flaps having fewer perforators[107,111,112].
A broad range of techniques for augmenting venous outflow in abdominally-based autologous breast reconstruction has been widely reported in the literature. The vena comitantes of the ipsilateral DIEA, vena comitantes of the contralateral DIEA, ipsilateral SCIV, ipsilateral SIEV, and contralateral SIEV serve as potential sources for donor veins in venous super-drainage, with the ipsilateral SIEV being the most common[113]. The most frequently used recipient vessel is the second internal mammary vein (if available), or the retrograde inframammary vein (IMV) due to their location allowing for optimal flap positioning on the chest. At our institution, for all DIEP flaps, we routinely preserve sufficient length on the SIEV and retrograde IMV to allow for anastomosis if necessary. Given the ease of supercharging the flap during the initial microsurgery, it is prudent to perform this anastomosis early if there is any level of concern for superficial venous dominance. Other potential recipients include the intercostal perforating vein, thoracodorsal vein, cephalic vein, thoracoacromial vein and lateral superficial thoracic vein - however, all of these systems require more time for dissection and may necessitate significant flap rotation to allow for anastomosis[113,114].
According to cadaveric and imaging studies, over 75 percent of females will have both lateral and medial vena comitantes to the inframammary artery (IMA) present above the lower border of the 4th intercostal space (ICS). If the lateral IMV is of adequate caliber for anastomosis, this may be used as the recipient vessel for venous supercharging[115]. Multiple studies have demonstrated that the caudal end of the IMV can accommodate retrograde flow, and have promoted the assumption that the IMV is valveless[116-119]. However, an anatomic study by Mackey and Ramsey on 32 human cadavers found that 1 to 3 valves were present in the IMV of 44% of female cadavers and 42% of male cadavers[120]. Additionally, valves were found between the preferred point of distal anastomosis and the next draining vein in 9% of 2nd ICS and 5% of 3rd ICS. While it is possible that valvular incompetence allowed for retrograde flow in prior studies, the findings by Mackey and Ramsey indicate that retrograde flow may not be guaranteed in the caudal end of the IMV, and that more dynamic studies are required to validate this technique[120]. In our experience, the lateral IMV is often diminutive, even if present. In these cases, we routinely preserve sufficient length on the retrograde end of the medial IMV and utilize this for secondary venous anastomosis. Although some authors may raise concern that retrograde outflow may be diminished due to the presence of valves, we have found that there is sufficient collateralization from the intercostal system and IMV perforators to allow for adequate outflow.
If no other recipient veins are available, it may be possible to perform venous turbo-drainage via an intra-flap anastomosis. Rohde and Keller have described a turbo-drainage technique in which a superficial to deep venous loop is created within the flap by anastomosing the ligated SIEV to the proximal end of one of the vena comitantes of the DIEA[121]. This allows blood from the superficial system to drain directly into the deep system via anterograde venous flow through the vena comitantes, and eventually through the original DIEV-IMV anastomosis. This technique requires minimal additional accommodations for vessel length or flap positioning. It is also suitable for cases in which the superficial venous system is overdominant.
If venous congestion persists despite venous outflow augmentation or there are no viable alternate recipient veins, mechanical leeching can be considered. This method entails intraoperatively placing an angiocatheter in the dominant vein, which is brought up to the skin as a venostomy for controlled manual drainage. The angiocatheter should be flushed periodically and aspirated at hourly intervals for the next 3-6 days based on clinical examination of the flap[122,123]. Bank et al. have also reported a case of successful resolution of venous congestion with mechanical leeching guided by ViOptix measurements[124]. Once congestion resolves, the angiocatheter can be removed and the vein can be allowed to clot. Although this method eliminates the infection risks associated with leech therapy, it still requires blood products due to volume depletion by aspiration. Furthermore, studies on mechanical leeching for venous congestion have so far reported high success rates, but given the limited number of studies and their small sample sizes, they are highly likely to be subject to publication bias.
Postoperative management of venous insufficiency
Delayed venous thrombosis in the postoperative period is most likely to occur within 3 days of the initial operation[125]. As previously discussed, early detection of a possible venous thrombosis allows for the best chance of ensuring flap survival. Since prolonged venous congestion is often a precursor to venous thrombosis, flap monitoring and timely detection of signs of venous congestion is essential to the prevention of this complication[104].
If clinical signs of venous congestion are present, initial management involves addressing sources of extrinsic compression that may be contributing to poor venous outflow. Common troubleshooting techniques include loosening of surgical bra, removing tight dressings, or removing compressive sutures[113,126-128]. Topical nitroglycerin paste causes both arterial and venous dilation; it may be used as an adjunct to remove extrinsic compression[127,129,130]. If conservative methods fail to relieve congestion, or if venous thrombosis is suspected, reoperation offers the best potential for flap salvage.
The strategy for addressing postoperative venous compromise in the operating room follows a pattern similar to that seen with intraoperative venous insufficiency. One notable exception is the presence of a hematoma that may be compressing the pedicle. Hematoma may be seen in the presence or absence of venous congestion[131]. However, it is more often seen concurrently with venous congestion. When hematoma is suspected as the cause of venous compromise, the hematoma should be evacuated on an emergent basis to avoid further compression of the pedicle[103,131]. In cases of delayed venous insufficiency (i.e., greater than 3 postoperative days) and/or when re-exploration of anastomosis and surgical revision may be impossible, venous insufficiency may be managed medically[128].
While the majority of venous thrombosis events occur within the immediate postoperative period, delayed venous insufficiency and/or thrombosis have been documented up to 5 weeks after initial operation[125,128]. In these later presentations, successful salvage without re-exploration of anastomosis is more common[128]. Yoon and Jones suggest a critical time period for flap survival whereby flaps with delayed thrombosis have a higher rate of survival due to neovascularization and angiogenesis that has already taken place[132].
Systemic anticoagulation in conjunction with reoperation
Heparin prevents clot formation by activating antithrombin III, which ultimately prevents the formation of fibrin[133]. While some have utilized antiplatelet therapy in addition to systemic anticoagulation, there is well-documented evidence to show that heparin is favorable to antiplatelet therapy in cases of microvascular thrombosis[134]. Several methods have been reported on the use of systemic heparin in cases of venous thrombosis or congestion, but timing, dosage, and routes of administration vary depending on the institution. Most authors report using the same protocol for systemic anticoagulation in both venous and arterial thrombotic complications. There is currently no standardized recommendation, and no single protocol has been proven to be superior. At our institution, we typically recommend a continuous weight-based heparin infusion titrated with a PTT of 60-80. This can be transitioned to weight-based low molecular weight heparin injections once the patient is deemed to have a sufficiently low bleeding risk. The duration of the treatment may range from 1 week to 4 weeks, depending on our level of concern for thrombosis.
Alternative venous drainage
Several methods exist for the medical management of venous insufficiency in free flaps, with varying levels of success demonstrated in the literature[127]. Local injection of subcutaneous heparin has been demonstrated to be effective in several studies. More recently, the use of subcutaneous heparin was discussed by Perez et al, who showed that local subcutaneous injection of LMWH is an effective method for flap salvage in cases of venous congestion[135].
Relief of venous congestion may be further facilitated by pricking or de-epithelialization of the flap. Pricking the flap with a needle allows blood loss from the congested area, thereby reducing venous compromise[136]. In a similar manner, de-epithelializing a portion of the flap allows for venous drainage. Heparin may be injected into the de-epithelialized area or a heparin-soaked gauze may be applied to the de-epithelialized area to further increase venous outflow[136,137].
Hirudotherapy, the use of medicinal leeches, may be used in cases of irreparable venous insufficiency and/or flap necrosis secondary to venous compromise. The application of leeches provides temporary relief of venous congestion while a more reliable network for venous drainage is being established[127,138]. The effectiveness of medicinal leech therapy in decreasing venous congestion is two-fold; the initial blood meal by the leech allows for active drainage of ~5-15 mL of congested blood, after which passive blood loss from the bite injury continues to occur. Leech-mediated release of vasoactive substances allows for further local thrombolysis and anticoagulation[139,140]. While leech therapy for free flap salvage has reported success rates ranging from 60-80%, it may be less effective for higher volume flaps such as TRAM or DIEP flaps[127,138]. Primary complications of leech therapy include infection and anemia[139]. The evidence for medicinal leech therapy is limited to case series and retrospective studies. While Pannucci et al. found that leech therapy in microvascular breast flaps was associated with higher flap loss rates, this is likely secondary to significant selection bias[141]. Current evidence indicates that leech therapy should be used with discretion and in consideration of patient-specific risk factors[141]. In our experience, leech therapy should be considered as an adjunct in cases with significant intra-flap venous insufficiency that does not respond adequately to other therapies.
Veno-cutaneous catheterization presents another option for the relief of venous congestion. An angiocatheter is placed into a superficial vein at the margin of the flap. Distilled heparin solution is injected into the vein. The catheter is left in place with a valve such that venous drainage may occur as needed. When clinical signs of congestion improve, the catheter may be removed[142-144]. In comparison to leech therapy, veno-cutaneous catheterization is less costly. Further, Mozafari et al. showed that veno-cutaneous catheter use is associated with decreased blood loss, wound dehiscence, and flap necrosis compared to leech therapy. It is also associated with high rates of nursing and patient satisfaction[145]. All reported protocols indicate that the catheter must be placed in the operating room, which is a notable disadvantage of this technique[127]. In our experience, venocutaneous catheterization can be difficult to maintain for more than 1-2 days, given the high likelihood of catheter thrombosis with intermittent use.
Negative pressure therapy has also been reported in the literature for the management of venous congestion. However, its use in practice is still rare. Negative pressure therapy is thought to reduce congestion by decreasing edema, increasing drainage and local venous flow, and increasing the rate of neovascularization[146-148]. Negative pressure may also have a compressive effect, making the overall benefit of this therapy difficult to accurately assess[127].
Special Considerations
Management of ischemia-reperfusion injury
Ischemia reperfusion injury is an important consideration for microsurgeons as tissue damage can persist well after the flow is re-established. Restoration of blood flow to a flap promotes the release of proinflammatory cytokines and reactive oxygen species (ROS), leading to tissue inflammation, coagulation, and necrosis. This cascade can ultimately result in partial or complete flap loss and fat necrosis as well as adverse patient outcomes and healthcare costs[149]. The most dreaded outcome in this scenario is the “no-reflow” phenomenon, whereby tissue damage is so severe that the flap does not perfuse despite the patency of the anastomosis. Several factors have been implicated in an increased risk for ischemia-reperfusion injury, including tissue type, surgical technique, temperature, and ischemia time[150].
Given the pathogenesis of ischemia-reperfusion injury, immunomodulators, antioxidants, and anticoagulants have each been proposed as potential therapeutics. While these therapies have shown promise in animal models, the data on their utility in human patients is unclear[151-157]. For example, while statins have theoretical anti-inflammatory and antioxidant activity, Koolen et al. and van den Heuvel et al. did not find such benefits in breast microsurgery[158,159]. Additionally, in a retrospective study, Coriddi et al. found no significant difference in lost vs salvaged flaps and patients who received intra/postoperative steroids or therapeutic anticoagulation for ischemia-reperfusion injury prophylaxis[103]. Ultimately, more research in this area, including randomized controlled clinical trials, is needed before further therapeutic recommendations are made.
When flap salvage is not feasible
When considering approaches to tertiary reconstruction, Baumeister et al. recommend the following steps: a sensitive psychosocial approach to the patient and family, an analysis of the cause of the first flap failure, reconsideration of the need for vascularized free tissue transfer, and a change in microsurgical strategy[160]. An investigation into the cause of flap failure should include careful consideration of the following: the preoperative preparations, the recipient vessels and anastomosis, the patient’s risk for hypercoagulability and thrombosis, the postoperative care, and the surgeon’s individual expertise. Baumeister et al. provide a thorough checklist to consider in [Table 2][160].
Checklist to be reviewed by surgeon after free flap failure. This figure is quoted from Baumeister et al.[160] published in Plastic & Reconstructive Surgery by the American Society of Plastic Surgeons, copyright 2008. from Wolters Kluwer Health, Inc
| Preoperative preparations |
| Did I know enough about the recipient vessels (artery and vein)? |
| Did I need an angiogram? |
| Did I adequately assess the patient’s coagulation potential? |
| Did I need to exclude a venous thrombosis? |
| Did I know about any previous operations, scars, or irradiation? |
| Were the type, size, and positioning of the flap properly planned? |
| Recipient vessels/anastomosis |
| Were there atherosclerotic changes? |
| Was there poor arterial outflow suggesting a proximal problem? |
| Did I need to perform the Fogarty maneuver on the artery? |
| Was it necessary to go more proximal using an interpositional graft to avoid the zone of injury? |
| Did I injure the vessel during preparation? |
| Was I satisfied with my technical performance during the anastomosis? Did I see every stitch? |
| Was it possible to improve the exposure of the vessels during anastomosis? |
| Was end-to-end or end-to-side anastomosis the best option? |
| Was there any tension or kinking of the vessels? |
| Did I irrigate with heparin? |
| Was there any vasospasm? |
| Should I have used papaverine or Xylocaine? |
| Was the room/patient warm enough? |
| Was the patient’s blood pressure adequate? |
| Were there any external constricting fascial bands or muscles compressing the vessels? |
| Coagulation/thrombosis |
| Was the operation performed in the acute posttraumatic period? |
| Was I satisfied with the coagulation of bleeding points? |
| Was there any thrombosis? |
| Intraoperative positioning |
| Were the exposure and approach to the vessels optimal? |
| Was it possible to operate in two teams and thus shorten the operating time? |
| Was it possible to improve the positioning of the surgeon during anastomosis? |
| Postoperative care |
| Was the patient hypovolemic, hypotonic, or hypothermic? |
| Was patient/flap positioning appropriate? |
| Was there any pressure on the proximal extremity/vessels? |
| Would it have been preferable to use an external fixator? |
| Was there pressure on the flap’s pedicle? |
| Were the flap’s perfusion and positioning adequately monitored (hourly)? |
| Would it have been preferable to use a Cook Doppler probe or a similar device? |
| Was the anticoagulation therapy adequate? Would full heparinization have helped? |
| Were there problems with patient compliance? |
| Revision |
| Was the thrombosis recognized early enough? |
| Was the revision performed immediately? |
| Would a different revision strategy have been preferable? |
| Surgeon |
| Would referral to another surgeon be appropriate? |
Hamdi et al. broadly classify the causes of flap failure into “technical” (anastomosis errors, pedicle kinking, anatomical variations, and quality and choice of recipient vessels and/or perforator of the nourishing pedicle) and “nontechnical” (one or more hypercoagulability disorders) etiologies[161]. In the event of “nontechnical” flap failure, alternative options, including a pedicled flap, should be strongly considered, given the high risk of another failure. However, for patients whose free flap failed due to a presumed technical error, another free flap may be reasonably considered.
In the rare case of non-salvageable total flap failure, an in-depth and empathetic discussion with the patient and family is essential. A description of possible alternative forms of breast reconstruction will provide necessary reassurance. We recommend debriding all non-viable tissue in the operating room soon after the diagnosis is assured. The mastectomy skin flaps should be closed if possible. If the skin flaps cannot be closed primarily, a negative pressure therapy dressing may be used temporarily. In a case of partial flap failure, debridement of the non-viable tissue should take place only after demarcation. The timing of future efforts at breast reconstruction should be dictated by the patient’s preferences, psychosocial needs, and the state of the wound after flap debridement.
Flap alternatives
Much of the decision-making regarding the next steps following flap failure depends on what was found following troubleshooting of the prior failed flap, anatomical limitations of the patient, and the patient’s preferences. The decision to pursue another reconstruction should be made only after a thorough reassessment of the patient’s medical and familial history for hypercoagulability and other potential risk factors. Following their review of 14 patients who underwent tertiary breast reconstruction after a prior failed reconstruction, Hamdi et al. recommend that, based on their experience, the latissimus dorsi flap and the thoracodorsal artery perforator flap with or without an implant are associated with lower morbidity compared to free flaps, and should be considered if the patient is at high risk of complications[161]. At our center, pedicled options such as the latissimus flap are essential for patients at high risk for microsurgical thrombosis. If the patient displays a strong preference for a free flap and they are deemed a candidate for a second attempt at free tissue transfer, preoperative planning should include CT and color Duplex imaging to assess alternative donor sites and viable recipient vessels, hematologic consultation for assessment of thromboembolism risk and application of thromboprophylaxis measures, and preparation of secondary options in case the second free flap fails.
CONCLUSION
Microvascular thrombosis continues to pose challenges in autologous breast reconstruction. Reconstructive surgeons should be mindful of obtaining relevant patient history, assessing risk factors, and consulting anatomical imaging when necessary during preoperative planning, and vigilantly monitor signs of flap compromise during the operative and postoperative phases. Cases of suspected thrombosis should be approached systematically to ensure proper management, using algorithms such as the ones we have presented in this review. Nevertheless, further investigation into individual techniques is necessary to optimize the prevention and management of thrombotic complications in breast reconstruction.
DECLARATION
Author’s contributions
Made substantial contributions to manuscript conception, literature review, manuscript preparation, and manuscript editing: Chen A, Garvey SR, Nanda A, Lee BT, Cauley RP
Availability of data and materials
Not applicable.
Financial support and sponsorship
None.
Conflicts of interest
All authors declare that there are no conflicts of interest.
Ethical approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Copyright
@ The Author(s) 2023.
REFERENCES
1. Durry A, Baratte A, Mathelin C, Bruant-Rodier C, Bodin F. [Patients' satisfaction after immediate breast reconstruction: comparison between five surgical techniques]. Ann Chir Plast Esthet 2019;64:217-23.
2. He WY, El Eter L, Yesantharao P, et al. Complications and patient-reported outcomes after tram and diep flaps: a systematic review and meta-analysis. Plast Reconstr Surg Glob Open 2020;8:e3120.
3. Khajuria A, Prokopenko M, Greenfield M, et al. A meta-analysis of clinical, patient-reported outcomes and cost of DIEP versus implant-based breast reconstruction. Plast Reconstr Surg Glob Open 2019;7:e2486.
4. Fischer JP, Sieber B, Nelson JA, et al. Comprehensive outcome and cost analysis of free tissue transfer for breast reconstruction: an experience with 1303 flaps. Plast Reconstr Surg 2013;131:195-203.
5. Fischer JP, Nelson JA, Cleveland E, et al. Breast reconstruction modality outcome study: a comparison of expander/implants and free flaps in select patients. Plast Reconstr Surg 2013;131:928-34.
6. Gill PS, Hunt JP, Guerra AB, et al. A 10-year retrospective review of 758 DIEP flaps for breast reconstruction. Plast Reconstr Surg 2004;113:1153-60.
7. Masoomi H, Clark EG, Paydar KZ, et al. Predictive risk factors of free flap thrombosis in breast reconstruction surgery. Microsurgery 2014;34:589-94.
9. Khansa I, Colakoglu S, Tomich DC, Nguyen MD, Lee BT. Factor V leiden associated with flap loss in microsurgical breast reconstruction. Microsurgery 2011;31:409-12.
10. Herrera FA, Lee CK, Kryger G, et al. Microsurgery in the hypercoagulable patient: review of the literature. J Reconstr Microsurg 2012;28:305-12.
11. Davison SP, Kessler CM, Al-Attar A. Microvascular free flap failure caused by unrecognized hypercoagulability. Plast Reconstr Surg 2009;124:490-5.
12. Wang TY, Serletti JM, Cuker A, et al. Free tissue transfer in the hypercoagulable patient: a review of 58 flaps. Plast Reconstr Surg 2012;129:443-53.
13. Pannucci CJ, Kovach SJ, Cuker A. Microsurgery and the hypercoagulable state: a hematologist's perspective. Plast Reconstr Surg 2015;136:545e-52e.
14. Friedman T, O'Brien Coon D, Michaels V J, et al. Hereditary coagulopathies: practical diagnosis and management for the plastic surgeon. Plast Reconstr Surg 2010;125:1544-52.
15. Nahabedian MY, Momen B, Galdino G, Manson PN. Breast reconstruction with the free TRAM or DIEP flap: patient selection, choice of flap, and outcome. Plast Reconstr Surg 2002;110:466-75.
16. Spear SL, Ducic I, Cuoco F, Taylor N. Effect of obesity on flap and donor-site complications in pedicled TRAM flap breast reconstruction. Plast Reconstr Surg 2007;119:788-95.
17. Hanwright PJ, Davila AA, Hirsch EM, et al. The differential effect of BMI on prosthetic versus autogenous breast reconstruction: a multivariate analysis of 12,986 patients. Breast 2013;22:938-45.
18. Chang DW, Wang B, Robb GL, et al. Effect of obesity on flap and donor-site complications in free transverse rectus abdominis myocutaneous flap breast reconstruction. Plast Reconstr Surg 2000;105:1640-8.
19. Fischer JP, Nelson JA, Sieber B, et al. Free tissue transfer in the obese patient: an outcome and cost analysis in 1258 consecutive abdominally based reconstructions. Plast Reconstr Surg 2013;131:681e-92e.
20. Chang DW, Wang B, Robb GL, et al. Effect of obesity on flap and donor-site complications in free transverse rectus abdominis myocutaneous flap breast reconstruction. Plast Reconstr Surg 2000;105:1640-8.
21. Heidekrueger PI, Fritschen U, Moellhoff N, et al. Impact of body mass index on free DIEP flap breast reconstruction: a multicenter cohort study. J Plast Reconstr Aesthet Surg 2021;74:1718-24.
22. Nolan J, Jenkins RA, Kurihara K, Schultz RC. The acute effects of cigarette smoke exposure on experimental skin flaps. Plast Reconstr Surg 1985;75:544-51.
23. Gu YD, Zhang GM, Zhang LY, Li FG, Jiang JF. Clinical and experimental studies of cigarette smoking in microvascular tissue transfers. Microsurgery 1993;14:391-7.
24. van Adrichem LN, Hoegen R, Hovius SE, et al. The effect of cigarette smoking on the survival of free vascularized and pedicled epigastric flaps in the rat. Plast Reconstr Surg 1996;97:86-96.
25. Khouri RK, Cooley BC, Kunselman AR, et al. A prospective study of microvascular free-flap surgery and outcome. Plast Reconstr Surg 1998;102:711-21.
26. Arnez ZM, Bajec J, Bardsley AF, Scamp T, Webster MH. Experience with 50 free TRAM flap breast reconstructions. Plast Reconstr Surg 1991;87:470-8.
27. Padubidri AN, Yetman R, Browne E, et al. Complications of postmastectomy breast reconstructions in smokers, ex-smokers, and nonsmokers. Plast Reconstr Surg 2001;107:342-9.
28. Baker DG, Krochak RJ. The response of the microvascular system to radiation: a review. Cancer Invest 1989;7:287-94.
29. Baker SR, Krause CJ, Panje WR. Radiation effects on microvascular anastomosis. Arch Otolaryngol 1978;104:103-7.
30. Arinci A, Topalan M, Aydin I, et al. Effects of early pre- and postoperative irradiation on the healing of microvascular anastomoses. J Reconstr Microsurg 2000;16:573-6.
31. Park JG, Yun HK, Ahn ST. The effect of radiation on the patency of end-to-side microvascular anastomosis. J Korean Soc Plast Reconstr Surg 2001:565-570. Available from: https://pesquisa.bvsalud.org/portal/resource/pt/wpr-70630 [Last accessed on 30 May 2023].
32. Barrera-Ochoa S, Gallardo-Calero I, López-Fernández A, et al. Effect of previous irradiation on vascular thrombosis of microsurgical anastomosis: a preclinical study in rats. Plast Reconstr Surg Glob Open 2016;4:e1073.
33. Fracol ME, Basta MN, Nelson JA, et al. Bilateral free flap breast reconstruction after unilateral radiation: comparing intraoperative vascular complications and postoperative outcomes in radiated versus nonradiated breasts. Ann Plast Surg 2016;76:311-4.
34. Fosnot J, Fischer JP, Smartt JM Jr, et al. Does previous chest wall irradiation increase vascular complications in free autologous breast reconstruction? Plast Reconstr Surg 2011;127:496-504.
35. Rogers NE, Allen RJ. Radiation effects on breast reconstruction with the deep inferior epigastric perforator flap. Plast Reconstr Surg 2002;109:1919-24.
36. Tran NV, Chang DW, Gupta A, Kroll SS, Robb GL. Comparison of immediate and delayed free TRAM flap breast reconstruction in patients receiving postmastectomy radiation therapy. Plast Reconstr Surg 2001;108:78-82.
37. Khavanin N, Yang JH, Colakoglu S, et al. Breast Reconstruction trends in the setting of postmastectomy radiation therapy: analysis of practices among plastic surgeons in the United States. Plast Reconstr Surg Glob Open 2023;11:e4800.
38. Lee M, Reinertsen E, McClure E, et al. Surgeon motivations behind the timing of breast reconstruction in patients requiring postmastectomy radiation therapy. J Plast Reconstr Aesthet Surg 2015;68:1536-42.
39. Baumann DP, Crosby MA, Selber JC, et al. Optimal timing of delayed free lower abdominal flap breast reconstruction after postmastectomy radiation therapy. Plast Reconstr Surg 2011;127:1100-6.
40. Meier CR, Jick H. Tamoxifen and risk of idiopathic venous thromboembolism. Br J Clin Pharmacol 1998;45:608-12.
41. Lin HF, Liao KF, Chang CM, Lin CL, Lai SW, Hsu CY. Correlation of the tamoxifen use with the increased risk of deep vein thrombosis and pulmonary embolism in elderly women with breast cancer: a case-control study. Medicine 2018;97:e12842.
42. Decensi A, Maisonneuve P, Rotmensz N, et al. Italian Tamoxifen Study Group. Effect of tamoxifen on venous thromboembolic events in a breast cancer prevention trial. Circulation 2005;111:650-6.
43. Debbie Jiang, Alfred Ian Lee. Thrombotic Risk from Chemotherapy and Other Cancer Therapies. In: Soff G, editor. Thrombosis and hemostasis in cancer. Cham: Springer International Publishing; 2019. pp. 87-101.
44. Kelley BP, Valero V, Yi M, Kronowitz SJ. Tamoxifen increases the risk of microvascular flap complications in patients undergoing microvascular breast reconstruction. Plast Reconstr Surg 2012;129:305-14.
45. Jokuszies A, Radtke C, Betzler C, et al. Is tamoxifen associated with an increased risk for thromboembolic complications in patients undergoing microvascular breast reconstruction? Ger Med Sci 2013;11:Doc05.
46. Parikh RP, Odom EB, Yu L, Colditz GA, Myckatyn TM. Complications and thromboembolic events associated with tamoxifen therapy in patients with breast cancer undergoing microvascular breast reconstruction: a systematic review and meta-analysis. Breast Cancer Res Treat 2017;163:1-10.
47. Tran BNN, Ruan QZ, Cohen JB, et al. Does Hormone Therapy Use Increase Perioperative Complications in Abdominally Based Microsurgical Breast Reconstruction? Plast Reconstr Surg 2018;141:805e-13e.
48. Mirzabeigi MN, Nelson JA, Fischer JP, et al. Tamoxifen (selective estrogen-receptor modulators) and aromatase inhibitors as potential perioperative thrombotic risk factors in free flap breast reconstruction. Plast Reconstr Surg 2015;135:670e-9e.
49. Billon R, Bosc R, Belkacemi Y, et al. Impact of adjuvant anti-estrogen therapies (tamoxifen and aromatase inhibitors) on perioperative outcomes of breast reconstruction. J Plast Reconstr Aesthet Surg 2017;70:1495-504.
50. Salibian AA, Bokarius AV, Gu J, et al. The effects of perioperative tamoxifen therapy on microvascular flap complications in transverse rectus abdominis myocutaneous/deep inferior epigastric perforator flap breast reconstruction. Ann Plast Surg 2016;77:630-4.
51. Ritter EF, Cronan JC, Rudner AM, Serafin D, Klitzman B. Improved microsurgical anastomotic patency with low molecular weight heparin. J Reconstr Microsurg 1998;14:331-6.
52. Zhang B, Dougan P, Wieslander JB. A comparison of the early antithrombotic effects between low molecular weight heparin and heparin in small arteries following a severe trauma: an experimental study. Ann Plast Surg 1993;31:255-61.
53. Lighthall JG, Cain R, Wieslander TA, Wax MK. Effect of postoperative aspirin on outcomes in microvascular free tissue transfer surgery. Otolaryngol Head Neck Surg 2013;148:40-6.
54. Zhou W, Zhang WB, Yu Y, et al. Are antithrombotic agents necessary for head and neck microvascular surgery? Int J Oral Maxillofac Surg 2019;48:869-74.
55. Chen CM, Ashjian P, Disa JJ, et al. Is the use of intraoperative heparin safe? Plast Reconstr Surg 2008;121:49e-53e.
56. Kroll SS, Miller MJ, Reece GP, et al. Anticoagulants and hematomas in free flap surgery. Plast Reconstr Surg 1995;96:643-7.
57. Ashjian P, Chen CM, Pusic A, et al. The effect of postoperative anticoagulation on microvascular thrombosis. Ann Plast Surg 2007;59:36-9.
58. Conrad MH, Adams WP Jr. Pharmacologic optimization of microsurgery in the new millennium. Plast Reconstr Surg 2001;108:2088-96.
59. Stephan B, Schenk JF, Nemeh A, Pindur G. The use of antithrombotic agents in microvascular surgery. Clin Hemorheol Microcirc 2009;43:51-6.
60. Brinkman JN, Derks LH, Klimek M, Mureau MA. Perioperative fluid management and use of vasoactive and antithrombotic agents in free flap surgery: a literature review and clinical recommendations. J Reconstr Microsurg 2013;29:357-66.
61. Liu J, Shi Q, Yang S, et al. Does postoperative anticoagulation therapy lead to a higher success rate for microvascular free-tissue transfer in the head and neck? a systematic review and meta-analysis. J Reconstr Microsurg 2018;34:87-94.
62. Microsurgery essentials: intra-operative technique. Available from: https://plasticsurgery.stanford.edu/education/microsurgery/intraoperative.html [Last accessed on 23 May 2023].
63. Krag C, Holck S. The value of the patency test in microvascular anastomosis: correlation between observed patency and size of intraluminal thrombus: an experimental study in rats. Br J Plast Surg 1981;34:64-6.
64. Phillips BT, Lanier ST, Conkling N, et al. Intraoperative perfusion techniques can accurately predict mastectomy skin flap necrosis in breast reconstruction: results of a prospective trial. Plast Reconstr Surg 2012;129:778e-88e.
65. Komorowska-Timek E, Gurtner GC. Intraoperative perfusion mapping with laser-assisted indocyanine green imaging can predict and prevent complications in immediate breast reconstruction. Plast Reconstr Surg 2010;125:1065-73.
66. Chatterjee A, Krishnan NM, Van Vliet MM, et al. A comparison of free autologous breast reconstruction with and without the use of laser-assisted indocyanine green angiography: a cost-effectiveness analysis. Plast Reconstr Surg 2013;131:693e-701e.
67. Lee BT, Matsui A, Hutteman M, et al. Intraoperative near-infrared fluorescence imaging in perforator flap reconstruction: current research and early clinical experience. J Reconstr Microsurg 2010;26:59-65.
68. Ludolph I, Horch RE, Arkudas A, Schmitz M. Enhancing safety in reconstructive microsurgery using intraoperative indocyanine green angiography. Front Surg 2019;6:39.
69. Yoshimatsu H, Karakawa R, Scaglioni MF, et al. Application of intraoperative indocyanine green angiography for detecting flap congestion in the use of free deep inferior epigastric perforator flaps for breast reconstruction. Microsurgery 2021;41:522-6.
70. Sharaf JM, Jacobs J, Henderson PW. Comments on "application of intraoperative indocyanine green angiography for detecting flap congestion in the use of free deep inferior epigastric perforator flaps for breast reconstruction". Microsurgery 2022;42:99-100.
71. Vijan SS, Tran VN. Microvascular breast reconstruction pedicle thrombosis: how long can we wait? Microsurgery 2007;27:544-7.
72. Mirzabeigi MN, Wang T, Kovach ST, et al. Free flap take-back following postoperative microvascular compromise: predicting salvage versus failure. Plast Reconstr Surg 2012;130:579-89.
73. Creech B, Miller S. Evaluation of circulation in skin flaps. In W.C. Grabb, M.B. Myers (Eds.), Skin Flaps. Boston: Little, Brown. 1975.
75. Jacobson A, Cohen O. Review of flap monitoring technology in 2020. Facial Plast Surg 2020;36:722-6.
77. Mericli AF, Wren J, Garvey PB, et al. A prospective clinical trial comparing visible light spectroscopy to handheld doppler for postoperative free tissue transfer monitoring. Plast Reconstr Surg 2017;140:604-13.
78. Smit JM, Zeebregts CJ, Acosta R, Werker PMN. Advancements in free flap monitoring in the last decade: a critical review. Plast Reconstr Surg 2010;125:177-85.
79. Ong AA, Ducic Y, Pipkorn P, Wax MK. Implantable doppler removal after free flap monitoring among head and neck microvascular surgeons. Laryngoscope 2022;132:554-9.
80. Karinja SJ, Lee BT. Advances in flap monitoring and impact of enhanced recovery protocols. J Surg Oncol 2018;118:758-67.
81. Lin SJ, Nguyen MD, Chen C, et al. Tissue oximetry monitoring in microsurgical breast reconstruction decreases flap loss and improves rate of flap salvage. Plast Reconstr Surg 2011;127:1080-5.
82. Pelletier A, Tseng C, Agarwal S, Park J, Song D. Cost analysis of near-infrared spectroscopy tissue oximetry for monitoring autologous free tissue breast reconstruction. J Reconstr Microsurg 2011;27:487-94.
83. Ricci JA, Vargas CR, Ho OA, et al. Evaluating the use of tissue oximetry to decrease intensive unit monitoring for free flap breast reconstruction. Ann Plast Surg 2017;79:42-6.
84. Lindelauf AAMA, Vranken NPA, Rutjens VGH, et al. Economic analysis of noninvasive tissue oximetry for postoperative monitoring of deep inferior epigastric perforator flap breast reconstruction: a review. Surg Innov 2020;27:534-42.
85. Ricci JA, Vargas CR, Lin SJ, et al. A novel free flap monitoring system using tissue oximetry with text message alerts. J Reconstr Microsurg 2016;32:415-20.
86. Zoccali G, Molina A, Farhadi J. Is long-term post-operative monitoring of microsurgical flaps still necessary? J Plast Reconstr Aesthet Surg 2017;70:996-1000.
87. Bonde C, Khorasani H, Eriksen K, et al. Introducing the fast track surgery principles can reduce length of stay after autologous breast reconstruction using free flaps: a case control study. J Plast Surg Hand Surg 2015;49:367-71.
88. Astanehe A, Temple-Oberle C, Nielsen M, et al. An enhanced recovery after surgery pathway for microvascular breast reconstruction is safe and effective. Plast Reconstr Surg Glob Open 2018;6:e1634.
89. Carruthers KH, Tiwari P, Yoshida S, Kocak E. Inpatient flap monitoring after deep inferior epigastric artery perforator flap breast reconstruction: how long is long enough? J Reconstr Microsurg 2019;35:682-7.
90. Khansa I, Chao AH, Taghizadeh M, et al. A systematic approach to emergent breast free flap takeback: Clinical outcomes, algorithm, and review of the literature. Microsurgery 2013;33:505-13.
91. Williams JG, French RJ, Lalonde DH. Why do free flap vessels thrombose? lessons learned from implantable doppler monitoring. Can J Plast Surg 2004;12:23-6.
92. Chen C, Nguyen MD, Bar-Meir E, et al. Effects of vasopressor administration on the outcomes of microsurgical breast reconstruction. Ann Plast Surg 2010;65:28-31.
93. Banic A, Krejci V, Erni D, Wheatley AM, Sigurdsson GH. Effects of sodium nitroprusside and phenylephrine on blood flow in free musculocutaneous flaps during general anesthesia. Anesthesiology 1999;90:147-55.
94. Massey MF, Gupta DK. The effects of systemic phenylephrine and epinephrine on pedicle artery and microvascular perfusion in a pig model of myoadipocutaneous rotational flaps. Plast Reconstr Surg 2007;120:1289-99.
95. Yu JT, Patel AJ, Malata CM. The use of topical vasodilators in microvascular surgery. J Plast Reconstr Aesthet Surg 2011;64:226-8.
96. Vargas CR, Iorio ML, Lee BT. A systematic review of topical vasodilators for the treatment of intraoperative vasospasm in reconstructive microsurgery. Plast Reconstr Surg 2015;136:411-22.
97. Ricci JA, Koolen PG, Shah J, et al. Comparing the outcomes of different agents to treat vasospasm at microsurgical anastomosis during the papaverine shortage. Plast Reconstr Surg 2016;138:401e-8e.
98. Ricci JA, Singhal D, Fukudome EY, et al. Topical nitroglycerin for the treatment of intraoperative microsurgical vasospasm. Microsurgery 2018;38:524-9.
99. Couteau C, Rem K, Guillier D, Moris V, Revol M, Cristofari S. Improving free-flap survival using intra-operative heparin: ritualistic practice or evidence-base medicine? a systematic review. Ann Chir Plast Esthet 2018;63:e1-5.
100. Troeltzsch M, Troeltzsch M, Probst FA, et al. Current concepts in salvage procedures for failing microvascular flaps: is there a superior technique? Int J Oral Maxillofac Surg 2016;45:1378-87.
101. Hong KY, Chang LS, Chang H, Minn KW, Jin US. Direct thrombectomy as a salvage technique in free flap breast reconstruction. Microsurgery 2017;37:402-5.
102. Kamali A, Docherty Skogh AC, Edsander Nord Å, et al. Increased salvage rates with early reexploration: a retrospective analysis of 547 free flap cases. J Plast Reconstr Aesthet Surg 2021;74:2479-85.
103. Coriddi M, Myers P, Mehrara B, et al. Management of postoperative microvascular compromise and ischemia reperfusion injury in breast reconstruction using autologous tissue transfer: retrospective review of 2103 flaps. Microsurgery 2022;42:109-16.
104. Tran NV, Buchel EW, Convery PA. Microvascular complications of DIEP flaps. Plast Reconstr Surg 2007;119:1397-405.
106. Nimalan N, Branford OA, Stocks G. Anaesthesia for free flap breast reconstruction. BJA Education 2016;16:162-6.
107. Blondeel PN, Arnstein M, Verstraete K, et al. Venous congestion and blood flow in free transverse rectus abdominis myocutaneous and deep inferior epigastric perforator flaps. Plast Reconstr Surg 2000;106:1295-9.
108. Heller L, Levin LS. Lower extremity microsurgical reconstruction. Plast Reconstr Surg 2001;108:1029-41.
109. Galanis C, Nguyen P, Koh J, et al. Microvascular lifeboats: a stepwise approach to intraoperative venous congestion in DIEP flap breast reconstruction. Plast Reconstr Surg 2014;134:20-7.
110. Wheatley MJ, Meltzer TR. The role of vascular pedicle thrombectomy in the management of compromised free tissue transfers. Ann Plast Surg 1996;36:360-4.
111. Kim DY, Lee TJ, Kim EK, Yun J, Eom JS. Intraoperative venous congestion in free transverse rectus abdominis musculocutaneous and deep inferior epigastric artery perforator flaps during breast reconstruction: A systematic review. Plast Surg 2015;23:255-9.
112. Baumann DP, Lin HY, Chevray PM. Perforator number predicts fat necrosis in a prospective analysis of breast reconstruction with free TRAM, DIEP, and SIEA flaps. Plast Reconstr Surg 2010;125:1335-41.
113. Ochoa O, Pisano S, Chrysopoulo M, Ledoux P, Arishita G, Nastala C. Salvage of intraoperative deep inferior epigastric perforator flap venous congestion with augmentation of venous outflow: flap morbidity and review of the literature. Plast Reconstr Surg Glob Open 2013;1:e52.
114. Enajat M, Rozen WM, Whitaker IS, Smit JM, Acosta R. A single center comparison of one versus two venous anastomoses in 564 consecutive DIEP flaps: investigating the effect on venous congestion and flap survival. Microsurgery 2010;30:185-91.
115. Seong IH, Woo KJ. Comparison of the second and third intercostal spaces regarding the use of internal mammary vessels as recipient vessels in DIEP flap breast reconstruction: An anatomical and clinical study. Arch Plast Surg 2020;47:333-9.
116. Kerr-Valentic MA, Gottlieb LJ, Agarwal JP. The retrograde limb of the internal mammary vein: an additional outflow option in DIEP flap breast reconstruction. Plast Reconstr Surg 2009;124:717-21.
117. Gravvanis A, Tsoutsos D, Papalois A, et al. The retrograde limb of the internal mammary vein in the swine model: a sufficient outflow option in free tissue transfer. Plast Reconstr Surg 2010;125:1298-9.
118. Li S, Mu L, Li Y, et al. Breast reconstruction with the free bipedicled inferior TRAM flap by anastomosis to the proximal and distal ends of the internal mammary vessels. J Reconstr Microsurg 2002;18:161-8.
119. Mohebali J, Gottlieb LJ, Agarwal JP. Further validation for use of the retrograde limb of the internal mammary vein in deep inferior epigastric perforator flap breast reconstruction using laser-assisted indocyanine green angiography. J Reconstr Microsurg 2010;26:131-5.
120. Mackey SP, Ramsey KW. Exploring the myth of the valveless internal mammary vein--a cadaveric study. J Plast Reconstr Aesthet Surg 2011;64:1174-9.
121. Rohde C, Keller A. Novel technique for venous augmentation in a free deep inferior epigastric perforator flap. Ann Plast Surg 2005;55:528-30.
122. Jindal R, Chong TW, Valerio IL, Gimbel ML, De La Cruz C. Alleviation of venous congestion in muscle-sparing free TRAM flaps with a temporary angiocatheter. Plast Reconstr Surg 2010;126:29e-31e.
123. Davies A, O'Neill JK, Wilson SM. Microvascular lifeboats: a stepwise approach to intraoperative venous congestion in DIEP flap breast reconstruction. Plast Reconstr Surg 2015;135:638e-9e.
124. Bank J, Beederman M, Shore AM, Song DH. Mechanical leeching with venocutaneous fistula and monitoring with near-infrared spectroscopy. Plast Reconstr Surg Glob Open 2013;1:e56.
125. Nelson JA, Kim EM, Eftakhari K, et al. Late venous thrombosis in free flap breast reconstruction: strategies for salvage after this real entity. Plast Reconstr Surg 2012;129:8e-15e.
126. Chaput B, Herlin C, Grolleau JL, Bertheuil N, Bekara F. Reply: the stitches could be the main risk for failure in perforator-pedicled flaps. Plast Reconstr Surg 2016;138:383e-5e.
127. Boissiere F, Gandolfi S, Riot S, et al. Flap venous congestion and salvage techniques: a systematic literature review. Plast Reconstr Surg Glob Open 2021;9:e3327.
128. Katira K, Goyal S, Venditto C, LoGiudice JA, Doren EL. Successful salvage of delayed venous congestion after diep flap breast reconstruction. Eplasty 2019;19:e22.
129. Gdalevitch P, Van Laeken N, Bahng S, et al. Effects of nitroglycerin ointment on mastectomy flap necrosis in immediate breast reconstruction: a randomized controlled trial. Plast Reconstr Surg 2015;135:1530-9.
130. Wang P, Gu L, Qin Z, Wang Q, Ma J. Efficacy and safety of topical nitroglycerin in the prevention of mastectomy flap necrosis: a systematic review and meta-analysis. Sci Rep 2020;10:6753.
131. Chu CK, Fang L, Kaplan J, Liu J, Hanasono MM, Yu P. The chicken or the egg? relationship between venous congestion and hematoma in free flaps. J Plast Reconstr Aesthet Surg 2020;73:1442-7.
132. Yoon AP, Jones NF. Critical time for neovascularization/angiogenesis to allow free flap survival after delayed postoperative anastomotic compromise without surgical intervention: a review of the literature. Microsurgery 2016;36:604-12.
133. Hanasono MM, Butler CE. Prevention and treatment of thrombosis in microvascular surgery. J Reconstr Microsurg 2008;24:305-14.
134. Khouri RK, Cooley BC, Kenna DM, Edstrom LE. Thrombosis of microvascular anastomoses in traumatized vessels: fibrin versus platelets. Plast Reconstr Surg 1990;86:110-7.
135. Pérez M, Sancho J, Ferrer C, García O, Barret JP. Management of flap venous congestion: the role of heparin local subcutaneous injection. J Plast Reconstr Aesthet Surg 2014;67:48-55.
137. Akan IM, Yildirim S, Gideroğlu K. Salvage of flaps with venous congestion. Ann Plast Surg 2001;46:456.
138. Knobloch K, Gohritz A, Busch K, Spies M, Vogt PM. [Hirudo medicinalis-leech applications in plastic and reconstructive microsurgery--a literature review]. Handchir Mikrochir Plast Chir 2007;39:103-7.
139. Hackenberger PN, Janis JE. A comprehensive review of medicinal leeches in plastic and reconstructive surgery. Plast Reconstr Surg Glob Open 2019;7:e2555.
140. Conforti ML, Connor NP, Heisey DM, Hartig GK. Evaluation of performance characteristics of the medicinal leech (Hirudo medicinalis) for the treatment of venous congestion. Plast Reconstr Surg 2002;109:228-35.
141. Pannucci CJ, Nelson JA, Chung CU, et al. Medicinal leeches for surgically uncorrectable venous congestion after free flap breast reconstruction. Microsurgery 2014;34:522-6.
142. Gürsoy K, Kankaya Y, Uysal A, Koçer U. Dealing with the venous congestion of free flaps: venous catheterization. J Craniofac Surg 2008;19:1645-7.
143. Eskitascioglu T, Coruh A, Ozyazgan I, Gunay GK. Salvage of venous congested flaps by simple methods. Plast Reconstr Surg 2006;117:344-6.
144. Caplin DA, Nathan CR, Couper SG. Salvage of TRAM flaps with compromised venous outflow. Plast Reconstr Surg 2000;106:400-1.
145. Mozafari N, Ghazisaidi MR, Hosseini SN, Abdolzadeh M. Comparisons of medicinal leech therapy with venous catheterization in the treatment of venous congestion of the sural flap. Microsurgery 2011;31:36-40.
146. Goldstein JA, Iorio ML, Brown B, Attinger CE. The use of negative pressure wound therapy for random local flaps at the ankle region. J Foot Ankle Surg 2010;49:513-6.
147. Qiu SS, Hsu CC, Hanna SA, et al. Negative pressure wound therapy for the management of flaps with venous congestion. Microsurgery 2016;36:467-73.
148. Uygur F, Duman H, Ulkür E, Ceiköz B. The role of the vacuum-assisted closure therapy in the salvage of venous congestion of the free flap: case report. Int Wound J 2008;5:50-3.
149. van den Heuvel MG, Buurman WA, Bast A, van der Hulst RR. Review: ischaemia-reperfusion injury in flap surgery. J Plast Reconstr Aesthet Surg 2009;62:721-6.
150. Marre D, Hontanilla B. Increments in ischaemia time induces microvascular complications in the DIEP flap for breast reconstruction. J Plast Reconstr Aesthet Surg 2013;66:80-6.
151. Im MJ, Manson PN, Bulkley GB, Hoopes JE. Effects of superoxide dismutase and allopurinol on the survival of acute island skin flaps. Ann Surg 1985;201:357-9.
152. Suzuki S, Yoshioka N, Isshiki N, Hamanaka H, Miyachi Y. Involvement of reactive oxygen species in post-ischaemic flap necrosis and its prevention by antioxidants. Br J Plast Surg 1991;44:130-4.
153. Aydogan H, Gurlek A, Parlakpinar H, et al. Beneficial effects of caffeic acid phenethyl ester (CAPE) on the ischaemia-reperfusion injury in rat skin flaps. J Plast Reconstr Aesthet Surg 2007;60:563-8.
154. Gurlek A, Celik M, Parlakpinar H, Aydogan H, Bay-Karabulut A. The protective effect of melatonin on ischemia-reperfusion injury in the groin (inferior epigastric) flap model in rats. J Pineal Res 2006;40:312-7.
155. Chappell D, Jacob M, Hofmann-Kiefer K, et al. Hydrocortisone preserves the vascular barrier by protecting the endothelial glycocalyx. Anesthesiology 2007;107:776-84.
156. Akdemir O, Hede Y, Zhang F, et al. Effects of taurine on reperfusion injury. J Plast Reconstr Aesthet Surg 2011;64:921-8.
157. Del Rio M, Lopez-Cabrera P, Malagón-López P, et al. Effect of intravenous lidocaine on ischemia-reperfusion injury in DIEP microsurgical breast reconstruction. a prospective double-blind randomized controlled clinical trial. J Plast Reconstr Aesthet Surg 2021;74:809-18.
158. Koolen PG, Nguyen JT, Ibrahim AM, et al. Effects of statins on ischemia-reperfusion complications in breast free flaps. J Surg Res 2014;190:378-84.
159. van den Heuvel MG, Bast A, Ambergen AW, van der Hulst RR. The effect of statins and other cardiovascular medication on ischemia-reperfusion damage in a human DIEP flap model: theoretical and epidemiological considerations. J Transplant 2012;2012:504081.
160. Baumeister S, Follmar KE, Zenn MR, Erdmann D, Levin LS. Strategy for reoperative free flaps after failure of a first flap. Plast Reconstr Surg 2008;122:962-71.
161. Hamdi M, Andrades P, Thiessen F, et al. Is a second free flap still an option in a failed free flap breast reconstruction? Plast Reconstr Surg 2010;126:375-84.
162. Um GT, Chang J, Louie O, et al. Implantable cook-swartz doppler probe versus synovis flow coupler for the post-operative monitoring of free flap breast reconstruction. J Plast Reconstr Aesthet Surg 2014;67:960-6.
163. Rozen WM, Chubb D, Whitaker IS, Acosta R. The efficacy of postoperative monitoring: a single surgeon comparison of clinical monitoring and the implantable Doppler probe in 547 consecutive free flaps. Microsurgery 2010;30:105-10.
164. Rozen WM, Chubb D, Whitaker IS, Acosta R. Postoperative monitoring of free flaps in autologous breast reconstruction: a multicenter comparison of 398 flaps using clinical monitoring, microdialysis, and the implantable Doppler probe. J Reconstr Microsurg 2010;26:409-16.
165. Smit JM, Whitaker IS, Liss AG, Audolfsson T, Kildal M, Acosta R. Post operative monitoring of microvascular breast reconstructions using the implantable Cook-Swartz doppler system: a study of 145 probes & technical discussion. J Plast Reconstr Aesthet Surg 2009;62:1286-92.
166. Schmulder A, Gur E, Zaretski A. Eight-year experience of the cook-swartz doppler in free-flap operations: microsurgical and reexploration results with regard to a wide spectrum of surgeries. Microsurgery 2011;31:1-6.
167. Chang EI, Ibrahim A, Zhang H, et al. Deciphering the sensitivity and specificity of the implantable doppler probe in free flap monitoring. Plast Reconstr Surg 2016;137:971-6.
168. Frost MW, Niumsawatt V, Rozen WM, et al. Direct comparison of postoperative monitoring of free flaps with microdialysis, implantable cook-swartz doppler probe, and clinical monitoring in 20 consecutive patients. Microsurgery 2015;35:262-71.
169. Kempton SJ, Poore SO, Chen JT, Afifi AM. Free flap monitoring using an implantable anastomotic venous flow coupler: analysis of 119 consecutive abdominal-based free flaps for breast reconstruction. Microsurgery 2015;35:337-44.
170. Jacob DD, Kwiecien GJ, Cakmakoglu C, Moreira A. Color Duplex ultrasound for localization of vascular compromise in microsurgical breast reconstruction. Plast Reconstr Surg 2020;145:666e-7e.
171. Arya R, Griffiths L, Figus A, et al. Post-operative assessment of perfusion of deep inferior epigastric perforator (DIEP) free flaps via pulsatility index (PI) using a portable colour doppler sonogram device. J Plast Reconstr Aesthet Surg 2013;66:931-6.
172. Rothenberger J, Amr A, Schaller HE, Rahmanian-Schwarz A. Evaluation of a non-invasive monitoring method for free flap breast reconstruction using laser doppler flowmetrie and tissue spectrophotometry. Microsurgery 2013;33:350-7.
173. Kumbasar DE, Hagiga A, Dawood O, Berner JE, Blackburn A. Monitoring Breast reconstruction flaps using near-infrared spectroscopy tissue oximetry. Plast Surg Nurs 2021;41:108-11.
174. Whitaker IS, Pratt GF, Rozen WM, et al. Near infrared spectroscopy for monitoring flap viability following breast reconstruction. J Reconstr Microsurg 2012;28:149-54.
175. Repez A, Oroszy D, Arnez ZM. Continuous postoperative monitoring of cutaneous free flaps using near infrared spectroscopy. J Plast Reconstr Aesthet Surg 2008;61:71-7.
176. Trignano E, Fallico N, Fiorot L, et al. Flap monitoring with continuous oxygen partial tension measurement in breast reconstructive surgery: a preliminary report. Microsurgery 2018;38:402-6.
177. Keller A. A new diagnostic algorithm for early prediction of vascular compromise in 208 microsurgical flaps using tissue oxygen saturation measurements. Ann Plast Surg 2009;62:538-43.
178. Koolen PGL, Vargas CR, Ho OA, et al. Does increased experience with tissue oximetry monitoring in microsurgical breast reconstruction lead to decreased flap loss? the learning effect. Plast Reconstr Surg 2016;137:1093-101.
179. Fox PM, Zeidler K, Carey J, Lee GK. White light spectroscopy for free flap monitoring. Microsurgery 2013;33:198-202.
180. Schrögendorfer KF, Nickl S, Keck M, et al. Viability of five different pre- and intraoperative imaging methods for autologous breast reconstruction. Eur Surg 2016;48:326-33.
Cite This Article
Export citation file: BibTeX | RIS
OAE Style
Chen A, Garvey SR, Nanda A, Lee BT, Cauley RP. Diagnosis, management and prevention of thrombotic complications in microsurgical breast reconstruction: a review of the literature. Plast Aesthet Res 2023;10:24. http://dx.doi.org/10.20517/2347-9264.2022.136
AMA Style
Chen A, Garvey SR, Nanda A, Lee BT, Cauley RP. Diagnosis, management and prevention of thrombotic complications in microsurgical breast reconstruction: a review of the literature. Plastic and Aesthetic Research. 2023; 10: 24. http://dx.doi.org/10.20517/2347-9264.2022.136
Chicago/Turabian Style
Chen, Amy, Shannon R. Garvey, Asha Nanda, Bernard T. Lee, Ryan P. Cauley. 2023. "Diagnosis, management and prevention of thrombotic complications in microsurgical breast reconstruction: a review of the literature" Plastic and Aesthetic Research. 10: 24. http://dx.doi.org/10.20517/2347-9264.2022.136
ACS Style
Chen, A.; Garvey SR.; Nanda A.; Lee BT.; Cauley RP. Diagnosis, management and prevention of thrombotic complications in microsurgical breast reconstruction: a review of the literature. Plast. Aesthet. Res. 2023, 10, 24. http://dx.doi.org/10.20517/2347-9264.2022.136
About This Article
Special Issue
Copyright
Data & Comments
Data

 Cite This Article 12 clicks
Cite This Article 12 clicks


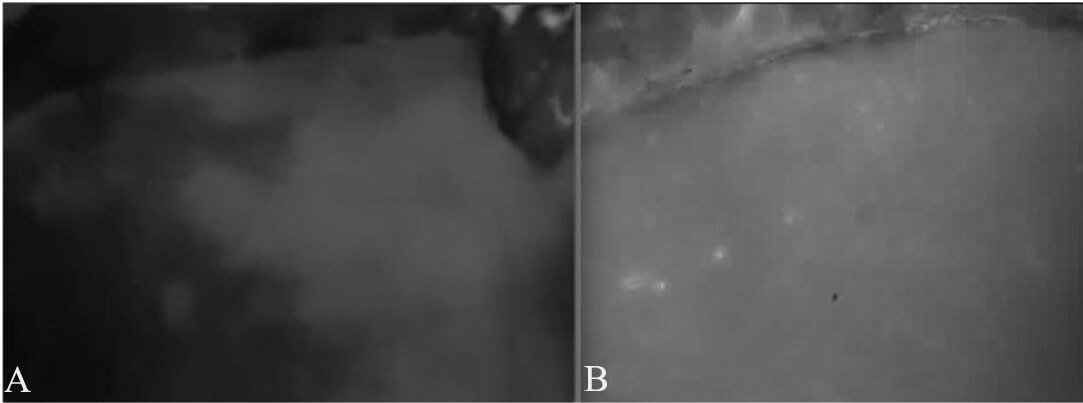
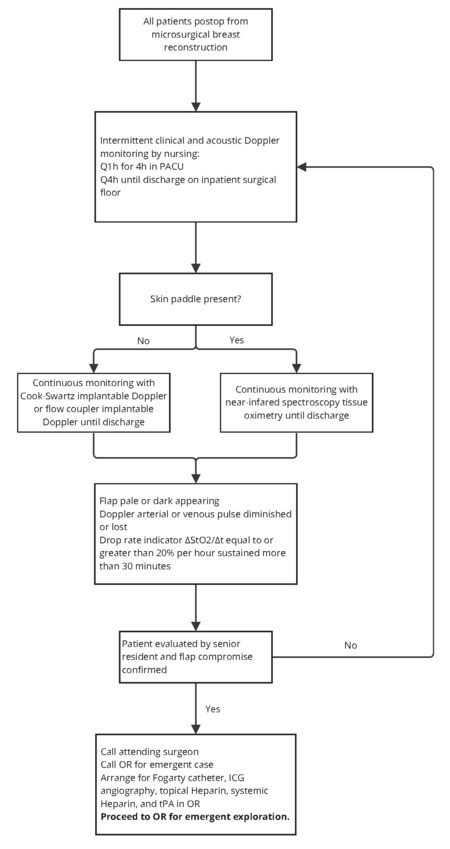
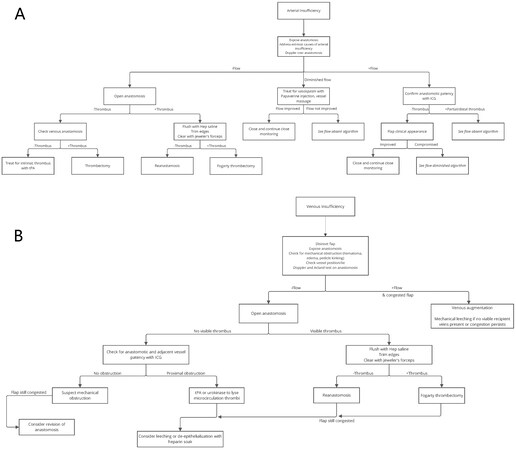
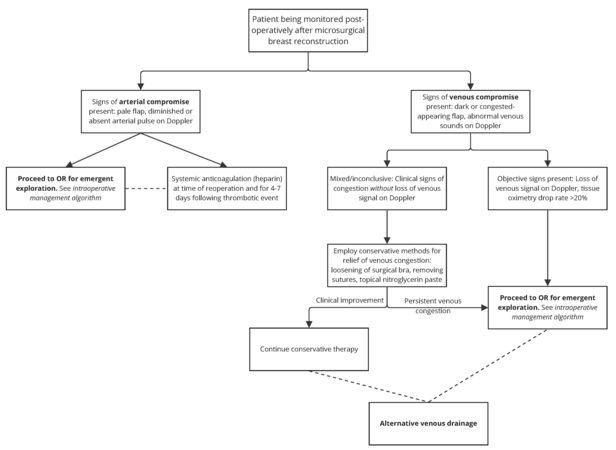








Comments
Comments must be written in English. Spam, offensive content, impersonation, and private information will not be permitted. If any comment is reported and identified as inappropriate content by OAE staff, the comment will be removed without notice. If you have any queries or need any help, please contact us at support@oaepublish.com.