Recent developments in metal nanocluster-based catalysts for improving photocatalytic CO2 reduction performance
Abstract
Photocatalytic reduction of carbon dioxide (CO2) is a promising technology for carbon recycling that offers both environmental and economic benefits. Among the potential photocatalysts, metal nanoclusters (MNCs) stand out as a class of materials with remarkable photophysical and photochemical properties. Despite the growing number of studies on MNCs-based photocatalytic reduction of CO2 in recent years, a systematic and comparative overview of these studies is still lacking. This review provides a concise and comprehensive summary of the latest research on MNCs-based catalysts for enhancing photocatalytic CO2 reduction performance. Moreover, this review highlights the challenges and opportunities in this field based on the current development trends.
Keywords
INTRODUCTION
Fossil fuels have been the dominant source of energy for various applications such as production, transportation, and power generation throughout human history. However, the excessive consumption of fossil fuels has led to the massive emission of carbon dioxide (CO2), which poses serious threats to energy security and environmental quality[1-9]. Numerous carbon fixation strategies have been developed to address these challenges, such as CO2 emission reduction, CO2 capture and storage (CCS), and CO2 utilization[10-12]. CO2 emission reduction involves the use of innovative technologies to lower the amount of CO2 produced during the production stage. However, these methods are often associated with high costs that are difficult for the general public to bear. The CCS also received significant attention, but the high cost and leakage risk limit its application on a large scale. CO2 utilization, in contrast, is the most attractive path. Chemical reforming, electrochemical reduction, biological reduction, and photochemical reduction are the leading CO2 utilization technologies. Photocatalytic CO2 reduction (PCR) is a sustainable process. It does not produce any toxic byproducts or cause any environmental pollution. PCR process can harness solar energy to reduce CO2 and produce valuable energy sources such as methane or methanol. Moreover, the PCR process is usually operating under mild conditions of ambient temperature and pressure. These advantages make PCR a highly desirable method for completing the carbon cycle[13-20].
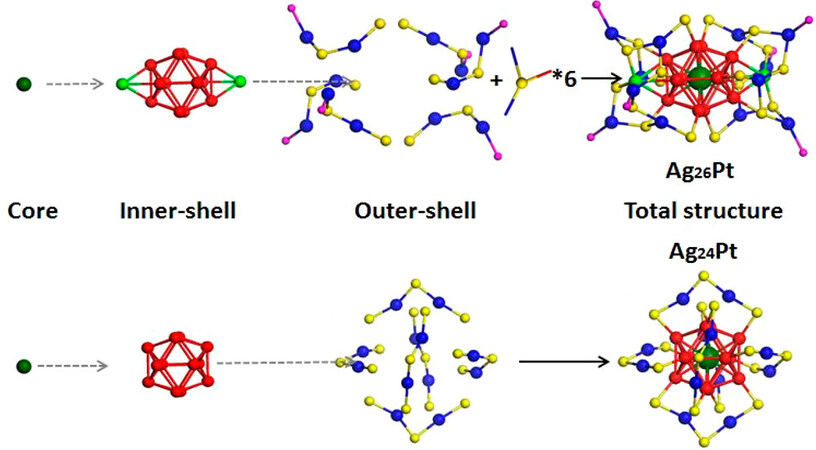
In the past few decades, the development of PCR catalysts has shifted from early bulky metals to nanostructured materials with specific properties[21]. Nanoclusters (NCs) are a new class of materials comprising a few to several hundred atoms surrounded by ligands. NCs have three components: the inner core, the outer atoms, and the surface ligands (as shown in Figure 1)[22]. NCs exhibit distinct physical and chemical properties compared to nanoparticles and molecules due to their distinctive electronic and geometric structures. The majority of the current NCs are metal nanoclusters (MNCs) with sizes ranging from 0.1 to 5 nm (typically < 2 nm for MNCs)[23], which is close to the Fermi wavelength (approximately
Figure 1. Structures of MNCs of Ag26Pt and Ag24Pt[22]. Copyright 2018, American Chemical Society.
Taking advantage of these inherent advantages, MNCs have been used as catalysts in various catalytic processes. For example, Kurashige et al. investigated Au NCs as co-catalysts for H2 evolution[26].
However, the synthesis and utilization of MNCs for PCR remain challenging. It requires a comprehensive understanding of their properties and catalytic reaction mechanisms. In this review, we provide a systematic overview of the recent advances in MNCs-based catalysts for PCR applications. We first introduce a general method for preparing MNCs. Next, we review how MNCs can enhance the PCR process as catalysts. We then classify the MNCs into two major categories: noble and nonnoble MNCs and summarize their advantages for PCR applications. Finally, we discuss the current challenges and prospects of MNCs for PCR. We hope this review will stimulate further research and innovation in this emerging field.
ADVANTAGES OF MNCS AND COMMON SYNTHESIS METHODS
Advantages of MNCs
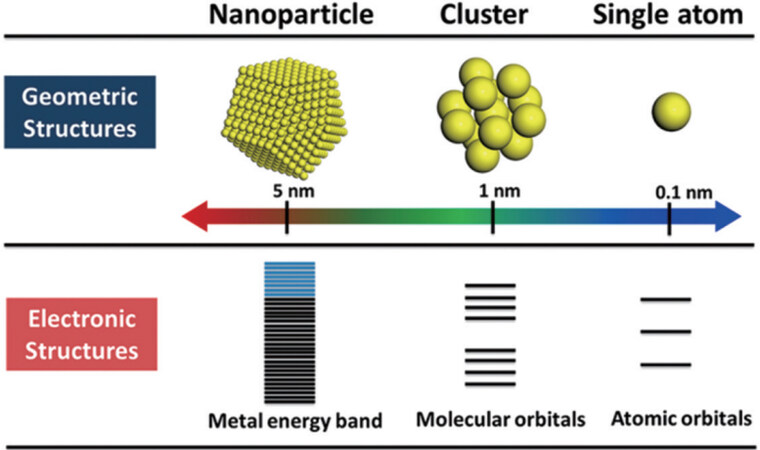
MNCs show a very different energy level structure in comparison with large-size nanoparticles (as shown in Figure 2). MNCs change the electron energy level near the Fermi level from quasi-continuous to discrete, resulting in energy level splitting or energy gap widening[32,33]. The discrete energy levels allow the electrons in MNCs to jump between energy levels and interact with light, which enhances the separation of electrons and holes[34]. Additionally, MNCs also have a small energy gap and absorb light in the visible and near-infrared regions[35,36]. Furthermore, MNCs are stable against oxidation or reduction by photogenerated electrons or holes[37,38]. More importantly, MNCs have a high specific surface area, a high fraction of low-coordinated atoms, a quantum size effect, a tunable composition, and a unique surface structure (e.g., pocket-like sites) due to their ultra-small size. These unique features of MNCs make them an emerging class of photocatalytic materials with increasing interest[39]. Moreover, their precise atomic-level structures enable fundamental research on the photocatalytic mechanisms, which provides theoretical guidance for the design of new photocatalytic materials and improves catalytic performance[40].
Figure 2. Geometric and electronic structures of single atoms, NCs, and nanoparticles[23]. Copyright 2018 American Chemical Society.
The synthesis methods of MNCs
There has been much research into the synthesis of MNCs, so we have summarized the commonly reported synthesis methods in the literature as follows[41]:
(1) Reduction growth method[42] (as shown in Figure 3 a→c): The metal core is created by reducing the respective metal ions. Therefore, the strength of the chosen reducing agent and the kinetic regulation of the reduction process are crucial for synthesizing the desired product.
Figure 3. Common methods for the synthesis of MNCs: (a→b) formation of M(I)-SR complexes, metal atom (M(I)), thiolate ligand (SR), (b→c) reduction growth, (c→d) seeded growth, (c→f) alloying reaction, (c→g) ligand-exchange process, (c→h) self-assembly of metal NCs, and (d→e) evolution from metal NCs to metal nanocrystals[41]. Copyright 2016, American Chemical Society.
(2) Seed growth method[43] (as shown in Figure 3 c→d): Smaller-sized MNCs are used as seeds to induce nucleation, resulting in the growth of larger-sized MNCs over time. The regulation of the crystal growth process is noteworthy.
(3) Alloying method[44] (as shown in Figure 3 c→f): The introduction of other metals for doping could be accomplished through the template exchange method, where the difference in electrode potential between the two metals serves as the driving force for the substitution reaction.
(4) Ligand exchange method (as shown in Figure 3 c→g): In principle, the ligand exchange method is similar to the alloying method in that both involve an exchange process, but the objects exchanged differ. For example, template exchange generates alloying NCs, whereas ligand exchange refers to preparing MNCs by exchanging the protective ligand layers on their periphery.
(5) The current atomically precise nano-chemistry is indeed moving toward the programmable synthesis of MNCs with control over the structure, such as the bcc, fcc, hcp, decahedra, icosahedra, multi-tetrahedral network, etc. (as shown in Figure 4)[45,46]. This programmable synthesis of MNCs offers promising opportunities for designing their structures and enhancing their catalytic performance[47].
Figure 4. Programmable atomic packing into different crystal structures of Au NCs[45]. Copyright 2021, Wiley-VCH.
THE PCR MECHANISM OVER MNCS-BASED PHOTOCATALYSTS
PCR involves multielectron reduction, and various products can be obtained via different reduction pathways, such as CO, CH4, HCOOH, C2H4, HCHO, and CH3OH. Below are the reduction potentials (E0, V vs. NHE) required for these products:[48]
CO2 + e- → CO2- E0 = -1.900 V (1)
CO2 + 2H+ + 2e- → HCOOH E0 = -0.610 V (2)
CO2 + 2H+ + 2e- → CO + H2O E0 = -0.530 V (3)
2CO2 + 2H+ + 2e- → H2C2O4 E0 = -0.913 V (4)
CO2 + 4H+ + 4e- → HCHO + H2O E0 = -0.480 V (5)
CO2 + 6H+ + 6e- → CH3OH+ H2O E0 = -0.380 V (6)
CO2 + 8H+ + 8e- → CH4+ 2H2O E0 = -0.240 V (7)
2CO2 + 12H+ + 12e- → C2H4+ 4H2O E0 = -0.349 V (8)
2CO2 + 14H+ + 14e- → C2H6+ 4H2O E0 = -0.270 V (9)
3CO2 + 18H+ + 18e- → C3H7OH + 5H2O E0 = -0.310 V (10)
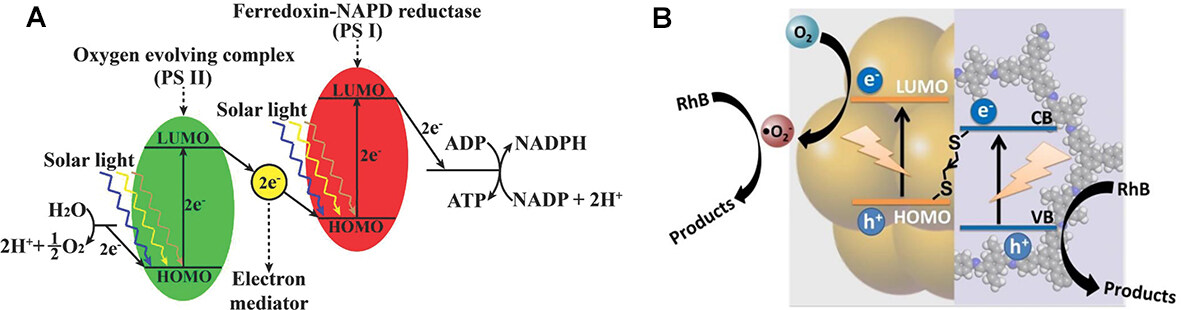
The ultra-small size of NCs gives them a strong quantum size effect, exhibiting discrete energy levels that allow electrons to undergo a leap from the highest occupied molecular orbital (HOMO) to the lowest unoccupied molecular orbital (LUMO), and electron-hole separation occurs. The energy gap of MNCs is typically less than 2.2 eV, allowing photocatalytic reactions under visible light irradiation. Therefore, MNCs can be considered semiconductor nanomaterials for photocatalytic reactions with small band gaps.
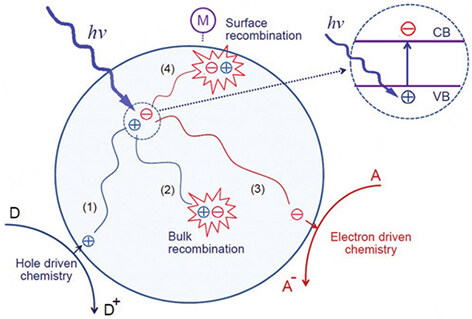
The mechanism of MNCs as catalysts in the PCR process is as follows (as shown in Figure 5)[49,50]. After irradiating the MNCs-based catalyst with light with photon energy equal to or greater than the band gap energy, the electrons transfer from the valence band (VB) to the conduction band (CB) to generate photogenerated electrons and holes. The photogenerated holes are transferred to the surface active sites for the oxidation reaction, as shown in process (1) of Figure 5[51]. In process (3) of Figure 5, photogenerated electrons are transferred to the surface-active site for the reduction reaction. Therefore, the photocatalytic performance is affected by the incident light absorption capacity and charge separation efficiency. To fully utilize the energy from solar radiation, the band gap of the photocatalyst should be below 3.1 eV to maximize light absorption[52]. Most of the solar radiation energy reaching Earth is in the visible spectrum. Additionally, the generated electrons and holes do not always migrate to the surface of the photocatalyst and react optimally. Occasionally, photogenerated electrons and holes will combine inside the catalyst [as shown in process (2) in Figure 5] or on the surface [as shown in process (4) in Figure 5] in a brief period, releasing energy in the form of heat or light that can result in a significant reduction in photocatalytic efficiency.
Figure 5. Photocatalytic process of MNCs[49]. Copyright 1995, American Chemical Society.
Therefore, the MNCs-based materials used to improve the PCR process must satisfy the following conditions: (1) Smaller energy gaps, typically exhibiting significant absorption in the visible or near-infrared region, make it easier to form photogenerated electron-hole pairs; (2) Good photostability, not readily oxidized or reduced by photogenerated holes or electrons. Long lifetimes of photogenerated electrons and holes so that the electron-hole pairs can participate more in redox reduction; and (3) The large specific surface area provides more catalytic sites, allowing free electrons to react with CO2 via charge transfer to the composite surface[53].
PROGRESS OF MNCS-BASED CATALYSTS IN PCR
MNCs have garnered attention for their potential to improve PCR performance due to their unique and desirable properties. Several reasons contribute to the focus on MNCs for PCR, including:
(1) High catalytic activity: The small size and high surface area-to-volume ratio of MNCs result in a high catalytic activity, which can enhance the efficiency of the PCR.
(2) Improved charge transfer: MNCs have been shown to improve the charge separation and transfer processes in photocatalytic systems, leading to higher photocatalytic activity.
(3) Tailor-made properties: The properties of MNCs can be easily tuned or customized by controlling the size, shape, and composition, allowing for better control of their catalytic activity.
Overall, MNCs have shown great potential for enhancing PCR performance. Continued research on MNCs can contribute to the development of sustainable and efficient CO2 reduction systems. Many strategies have been proposed to use MNCs as catalysts for improving PCR performance. This section reviews the recent advances in MNCs-based catalysts for enhancing PCR performance. We classify the MNCs-based catalysts into two subsections, nonnoble (e.g., Cu[54], CdS[55], Fe, Co[56], Ni, Cu) and noble MNCs-based (e.g., Au[57], Ag[58], Pt) catalysts[59]. Then, we describe their respective roles as catalysts for improving the PCR process. The photocatalytic performance of various MNCs-based catalysts under visible-light irradiation is presented in Table 1.
Performance of various noble & nonnoble MNCs used for PCR
| Sample | Performance | References |
| Cu (II)-TiO2 | CO2 generation rate = 0.13 µmol/h; quantum efficiency = 27.7% | [67] |
| Cu(II)-Nb-doped TiO2 | CO2 generation rate = 0.20 µmol/h; quantum efficiency = 25.3% | [69] |
| Cu(II)-TiO2 after the coordination of oxygen and metal | CO2 generation rate = 0.40 µmol/h; quantum efficiency = 68.7% | [75] |
| Fe(III)-TiO2 | CO2 generation rate = 0.40 µmol/h; quantum efficiency = 53.5% | [77] |
| Fe(III)-Ti(IV)-TiO2 | CO2 generation rate = 0.69 µmol/h; quantum efficiency = 92.2% | [79] |
| Ni/TiO2 | CH3CHO production rate = 1.42 µmol g-cat-1 | [81] |
| TiO2-x/CoOx | CO production rate = 1.2473 µmol/g/h; CH4 production rate = 0.0903 µmol/g/h | [82] |
| CeOx-S/ZnIn2S4 | CO productivity of 1.8 mmol/g/ with a rate of 0.18 mmol/g/h | [83] |
| Au NCs/TiO2/Ti3C2 with CBD method | CO yield of 27.5 µmol/g in 3 h; CH4 yield of 42.11 µmol/g in 3 h | [87] |
| SNO/CdSe–DET | CO production rate = 36.16 µmol/g/h | [102] |
| Au-NC@UiO-68-NHC | CO production rate = 57.57 µmol/g/h | [107] |
Nonnoble MNCs-based catalysts in PCR
Nonnoble MNCs offer a wide range of material selection options, and commonly available and inexpensive metals such as Fe, Ni, Cu, and Al can be used to prepare MNCs. Additionally, they are generally less costly compared to noble MNCs, resulting in lower preparation costs. In addition, semiconductor materials with wide energy gaps, such as TiO2, SrTiO3, BiVO4, Ta3N5, g-C3N4, CdS, and MoS2, are the most widely used photocatalysts[60]. However, most required light sources are in the ultraviolet spectrum, with low solar utilization and high electron-hole complexation rates, resulting in low photocatalytic reaction efficiency[61-63]. Consequently, using nonnoble MNCs as co-catalysts is suitable for overcoming these disadvantages[64]. Due to the advantages mentioned above, nonnoble MNCs-based catalysts are primarily employed for surface modification of semiconductor materials to enhance PCR performance. The reaction process for nonnoble MNCs-based catalysts to improve PCR performance by surface modification techniques has two steps, namely visible-light-induced interfacial charge transfer (IFCT) between the MNCs and semiconductor materials and the multielectron reduction of oxygen molecules mediated by the co-catalytic promoter effect of the MNCs[65].
Preparation of nonnoble MNCs-based catalysts by impregnation to enhance PCR performance
Photocatalysts composed of nonnoble MNCs and semiconducting materials are typically fabricated by the impregnation method[66]. Impregnation is a method of incorporating one material into another by soaking or spraying the material onto the surface of the other. Impregnation involves the following steps: The prepared semiconducting material is soaked or sprayed with the MNCs’ solvent, which contains a suspension of MNCs, and allowed to dry. The MNCs are then deposited onto the surface of the semiconducting material. The impregnated semiconducting material is then calcined at high temperatures to remove the solvent and stabilize the MNCs on the surface of the semiconducting material. The impregnation method allows for the controlled deposition of MNCs onto the surface of semiconducting materials, thereby preparing materials with good photocatalytic activity.
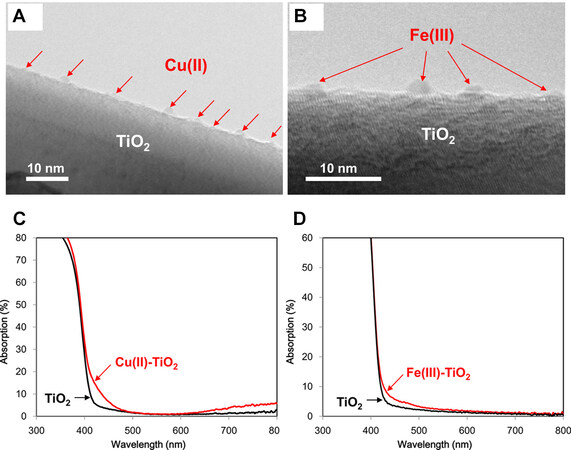
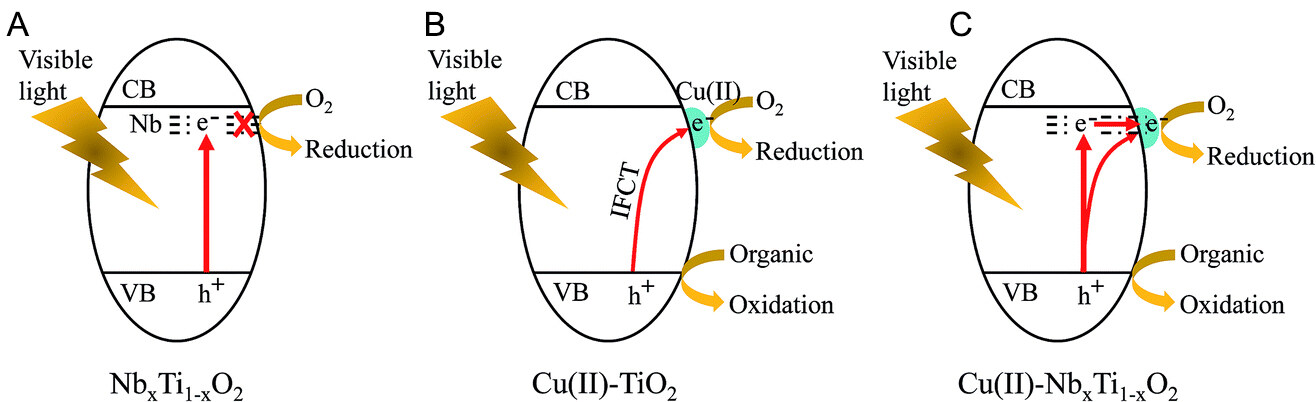
The initial focus of the research was to improve the visible light sensitivity of TiO2 semiconductors by using Cu(II) or Fe(III) NCs as co-catalysts (as shown in Figure 6)[67,68]. However, this photocatalytic system has limited catalytic efficiency because IFCT occurs only at the TiO2/MNCs interface. Liu et al. designed a TiO2 photocatalyst that can respond to visible light based on the principle of energy level matching[69]. The energy level occupied by the N-doped particles below the conduction band of TiO2 matches the potential of the Cu2+/Cu+ redox couple in the Cu(II) NCs. The matched energy levels facilitate the efficient transfer of photogenerated electrons from the doped Nb state to the Cu(II) NCs, thereby contributing to the efficient multielectron reduction of oxygen molecules (as shown in Figure 7)[69,70]. This method provides a practical and strategic approach to creating new MNCs materials with effective photocatalytic properties.
Figure 6. Cu (II) (A) and Fe (III) (B) NCs-grafted TiO2 images captured by TEM. UV-Vis absorption spectra for Cu(II) (C) and Fe(III) (D) NCs-grafted TiO2[70]. Copyright 2016, American Chemical Society.
Figure 7. Proposed photocatalytic processes for (A) NbxTi1-xO2, (B) Cu(II)-TiO2, and (C) Cu(II)-NbxTi1-xO2, respectively[70]. Copyright 2016, American Chemical Society.
Nonnoble MNCs-based catalysts improve PCR performance by increasing vacancies and defects
Nonnoble MNCs-based catalysts are not limited to simple grafting modifications as co-catalysts. It was discovered that the performance of PCR could be enhanced by increasing the vacancies and defects. By generating oxygen vacancies, these modifications increase the surface negative charge density[71]. Upon exposure to light, the oxygen vacancies accumulate additional negative charges that contribute to the extension of the visible light absorption of semiconductor material, making the metal oxide capable of activating CO2[72].
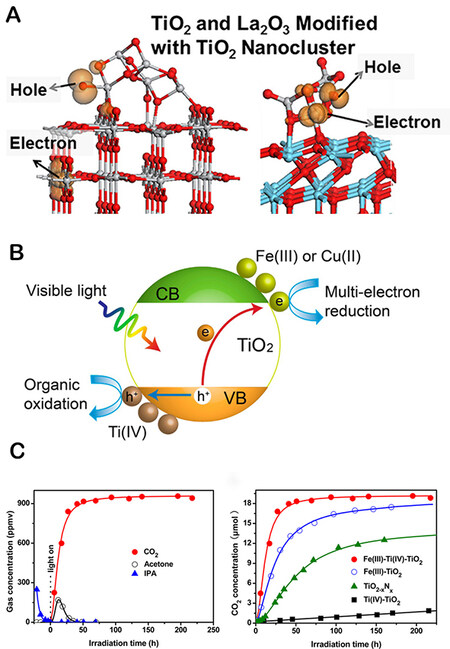
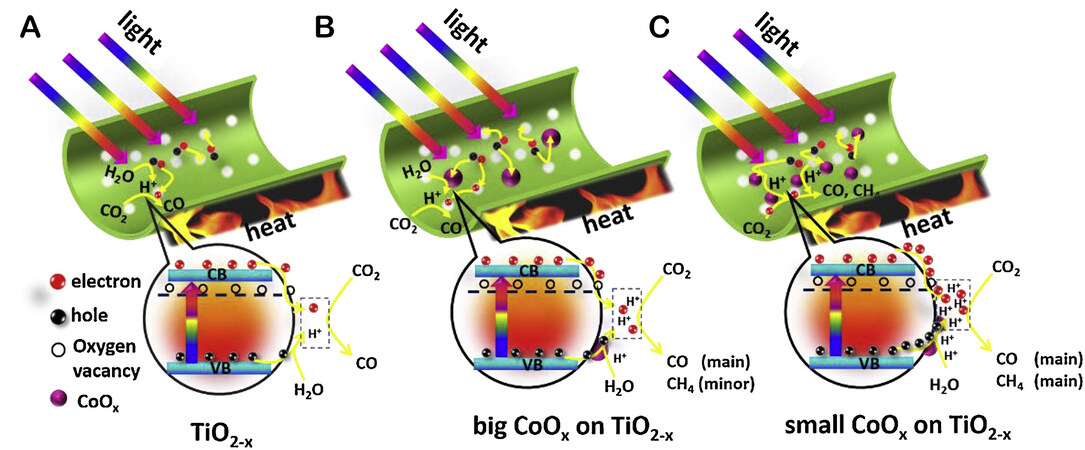
Nolan et al. present a study of electron and hole localization in low-coordinated titanium and oxygen sites of free and metal oxide-supported TiO2 nanocrystals (as shown in Figure 8A)[73]. This approach highlights how nonnoble MNCs can enhance oxygen and metal coordination by modifying semiconductor materials. The structure of MNCs as catalysts in semiconductors reveals a significantly different metal and oxygen coordination environment compared to that of the unmodified semiconductor[74,75]. Low-coordinated metal and oxygen sites are crucial as charge carrier capture sites and active sites for target molecules, such as carbon dioxide and water[76]. Thus, Liu et al. developed a more sophisticated synthesis strategy by employing MNCs with poorly coordinated metal and oxygen sites as catalysts[77,78]. Liu et al. also demonstrated that amorphous Ti(IV) NCs promoted the oxidation of organic compounds effectively (as shown in Figure 8B) and that TiO2 with Fe(III) and Ti(IV) NCs as catalysts achieved a Q.E. of 90% (as shown in Figure 8C)[79]. Additionally, Cheng et al. recently published the first study on Cu clusters mediated into Cd vacancies at the edges of CdS nanorods for photocatalytic CO2 conversion[80]. Billo et al. reported a Ni-NCs/TiO2 catalyst with improved PCR performance[81]. The Ni-NCs and O vacancies provide energetically stable CO2 binding sites for CO2 reduction, allowing for rapid electron transport for enhanced solar energy harvesting[81]. This method enhances the photocatalytic activity and selectivity of Ni/TiO2 via a synergistic interaction in which the active center increases activity by lowering the activation barrier energy for CO2 dissociation, and CO2 molecules can bind to Ni and defect sites. Li et al. developed an effective photothermal catalyst by modifying TiO2 nanotubes with a minute amount of CoOx and oxygen vacancies. The results demonstrated that introducing oxygen vacancies facilitated the charge separation and dispersion of CoOx co-catalysts, in which grafted CoOx acted as hole traps and promoted the release of more protons (as shown in Figure 9)[82]. In addition, Hou et al. have significantly enhanced the activity by constructing CeOx NCs with surface defect sites via a “partial sulfation” technique. The underlying principle of this strategy is improving the surface electronic properties of CeOx-S NCs, which in turn induces the appearance of several Ce3+ and oxygen vacancies[83]. The photogenerated electrons were captured by oxygen vacancies on the
Figure 9. Scheme of photothermocatalytic reaction over three typical samples: (A) TiO2-x. (B) big CoOx clusters modified TiO2-x.
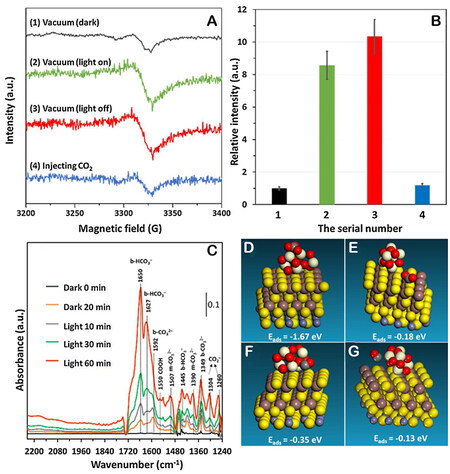
Figure 10. Investigation of the promotional role of CeOx-S clusters in electron transfer and the subsequent surface reaction. (A) In situ EPR signals of CeOx-S/ZnIn2S4. (B) Quantitative analysis by the double integral of the EPR signals in a. (C) In situ FTIR spectra for the adsorption, activation, and reduction of CO2 under visible light over CeOx-S/ZnIn2S4. (D and E) Adsorption energies of CO2 and
Noble MNCs-based catalysts in PCR
While the high cost of noble MNCs limits their use in large quantities, they possess large energy bandwidths and high electron densities that enable them to rapidly receive and release electrons. This results in the high catalytic activity of noble MNCs[84]. Noble MNCs are also known to exhibit unique electrical and thermodynamic properties, which allow them to perform reactions that other catalysts cannot. For instance, they are being utilized in biological research.
Noble MNCs-based catalysts improve PCR performance by cluster beam deposition
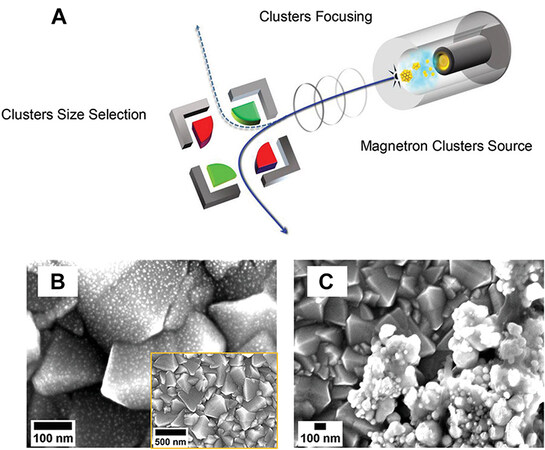
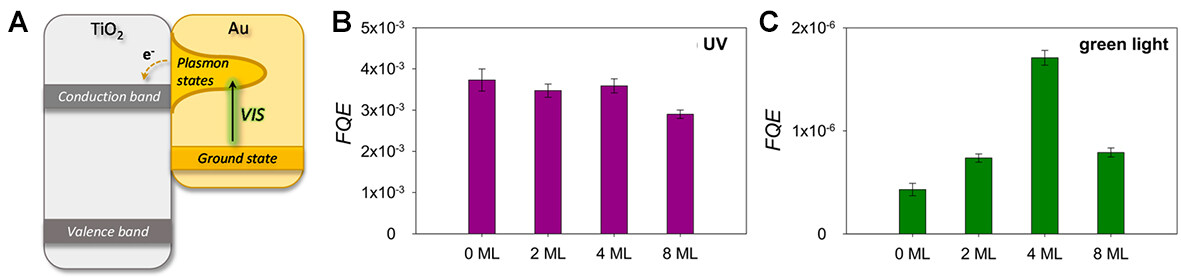
The acids and ligands used in conventional surface modification techniques have a chemical contamination effect on the MNCs (the effect causes the change of a single atom to have a significant physical and chemical effect on the MNCs). Cluster beam deposition (CBD) was proposed to avoid the chemical contamination of MNCs. CBD produces cluster-modified semiconductor materials with exceptional photocatalytic properties by forming clusters in a gaseous environment and then soft-landing them on supports with precise control over their size, shape, and composition (as shown in Figure 11)[85]. After deposition, unlike wet chemistry, no additional calcination or activation steps are required[86]. The vapor-phase Au NCs deposition method permits the placement of all active particles on the surface. Li et al. also prepared Au NCs/TiO2/Ti3C2 and Au nanoparticles/TiO2/Ti3C2 by deposition and precipitation[87]. The optimized Au NCs/TiO2/Ti3C2 exhibited higher yields of reduced CO2 to CO and CH4 than Au-nanoparticles/TiO2/TiCO2 and P-Au-nanoparticles/TiO2/TiCO2 (as shown in Figure 12)[87]. CBD for MNCs synthesis not only reduces the risk of chemical contamination but also ensures that the MNCs are evenly distributed over the semiconductor surface and reduces costs.
Figure 11. (A) Schematic illustration of magnetron CBD technology. High-Resolution SEM images of (B) Au NCs modified FTO and (C) colloidal Au modified FTO. The inset image in (B) shows the global view of deposited Au NCs[85]. Copyright 2021, Wiley-VCH.
Figure 12. (A) Schematic illustration of hot electron transfer from an excited plasmonic state on the gold nanoparticle to the TiO2 conduction band. Formal Quantum Efficiency (FQE) under (B) UV and (C) green light illumination as a function of Au NCs coverage on TiO2 P25[86]. Copyright 2018, Wiley-VCH.
Preparation of Noble MNCs-based catalysts by preventing clusters from aggregating improve PCR performance
Due to their large specific surface area and high surface energy, noble MNCs are typically thermodynamically unstable and susceptible to migration and agglomeration under light irradiation or high-temperature conditions, resulting in a substantial decrease in catalytic activity and selectivity[88,89]. Therefore, strategies to prevent cluster aggregation on the carrier are necessary to enhance their photocatalytic performance[90].
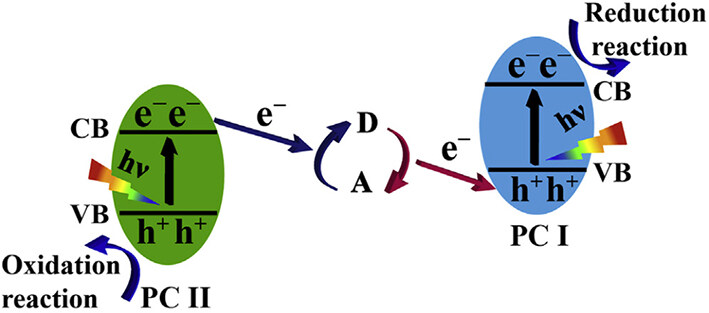
Bard proposed traditional Z-scheme photocatalysts in 1979[91], which can improve charge separation efficiency and retain strong redox capabilities. This system consists of two semiconducting materials with appropriate intermediate couples, such as Fe3+/Fe2+, IO3-/I-, and I3-/I-[92]. These two semiconductors have band structure configurations that differ. In a perfect process, photogenerated holes in the VB of PC I react with electron donors to produce electron acceptors. Photogenerated electrons in PC II’s CB react with electron acceptors to produce electron donors. Then, photogenerated electrons in PC I’s CB and holes in PC II’s VB participate in the reduction and oxidation reactions, respectively (as shown in Figure 13)[93,94]. This mode of charge transfer can endow this system with powerful redox capability and spatially distinct redox reaction sites. Deng et al. developed PCR catalysts based on Z-scheme Au NCs (as shown in Figure 14)[95]. By combining with the photogenerated electrons in the coupled semiconductor, the photogenerated holes in the Au NCs can be consumed. This combination prevents the self-oxidative aggregation of Au NCs and increases their stability, thereby enhancing their photocatalytic activity[95]. Therefore, it is essential to combine NCs with suitable semiconductors when constructing a Z-scheme heterojunction system.
Figure 13. Schematic illustration of charge transfer in traditional Z-scheme heterojunction photocatalysts. D in the figure indicates electron donors, and A in the figure indicates electron acceptors[94]. Copyright 2020, Elsevier Inc.
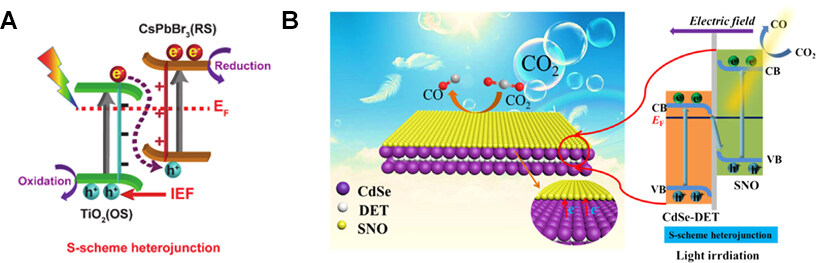
In addition to Z-scheme heterojunction structures, S-scheme heterojunctions are utilized to fabricate catalysts for improving PCR performance. (as shown in Figure 15A)[96]. S-scheme heterojunction has the following advantages: (1) the photocatalytic system can have both a wide photo-response range and a strong redox ability; (2) the large internal contact area and the rapid separation of carriers in the S-scheme system suppress the photo-induced electron-hole pair combination, which further improves the photocatalytic ability[97-101]. Ke et al. initially fabricated a novel S-scheme SNO/CdSe-DET composite and investigated its PCR activity (as shown in Figure 15B)[102]. The SNO/CdSe-DET composites exhibited excellent CO2 photoreduction stability. Such a superior activity should be ascribed to the S-scheme system, which benefits the separation of the photogenerated carriers and promotes the synergy between CdSe-DET nanorods and SNO nanosheets by strong chemical-bonding coordination.
Figure 15. (A) S-scheme heterojunction. (B) The S-scheme SNO/CdSe-DET heterojunction charge migration and separation diagram under light irradiation[102]. Copyright 2021, Wiley-VCH.
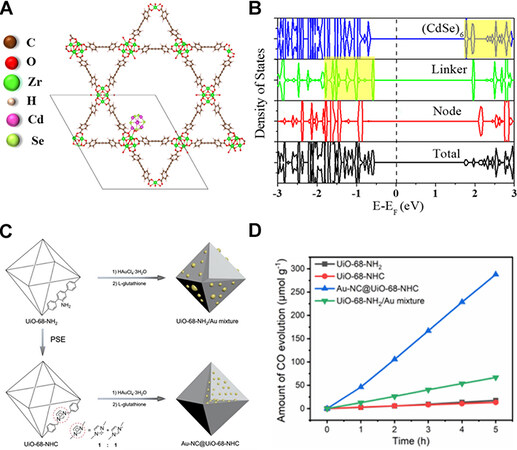
The encapsulation of MNCs in metal-organic frameworks (MOFs) has also garnered considerable interest[103,104]. By encapsulating MNCs within the environment of the MOFs structure, their fluctuations and aggregation can be effectively reduced, resulting in improved stability and catalytic efficiency. The MOFs structure prevents MNCs from interacting with undesirable species in the reaction environment, which can have a detrimental impact on their performance. Furthermore, the highly porous and interconnected structure of MOFs enables the efficient mass transfer of molecules to and from the active sites of MNCs, thereby enhancing their catalytic activity[105]. Overall, the MOFs-encapsulated MNCs show enhanced stability and improved performance, making them highly attractive for improving PCR performance. Indrani Choudhuri and Donald G. Truhlar studied a composite material containing a Cd6Se6 cluster in the pore of NU-1000 MOF. The Cd6Se6@NU-1000 composite permits electron transfer from the visible-light photo-excited organic linker to the lowest unoccupied orbital of the inorganic cluster, which can result in charge separation (as shown in Figure 16A and B)[106]. Jiang et al. present a heterogeneous nucleation strategy for stabilizing and dispersing ultrasmall Au NCs in an NHC-functionalized porous matrix (as shown in Figure 16C)[107]. The Au NCs are embedded in the MOF material to prevent cluster aggregation. It gives the composite material a high degree of photostability and chemical stability. During the PCR process, the Au-NC@MOF composite demonstrates outstanding and consistent activity (as shown in
Figure 16. (A) Structure of the Cd6Se6 cluster encapsulated in NU-1000 at a node site (Cd6Se6@NU-1000). The unit cell of the system is denoted by a solid line. (B) Total and partial density of states of Cd6Se6@NU-1000. The Fermi level is set to zero and denoted by a black dashed line. The highest occupied crystal orbital (HOCO) is on the linker, and the lowest unoccupied crystal orbital (LUCO) is on the cluster. The highest-energy occupied orbitals on the linker and the lowest-energy unoccupied orbitals on the cluster are highlighted in yellow[106]. Copyright 2020, American Chemical Society (C) Schematic presentation for the synthesis of UiO-68-NHC,
Noble MNCs-based catalysts improve PCR performance by photosynthetic biohybrid system
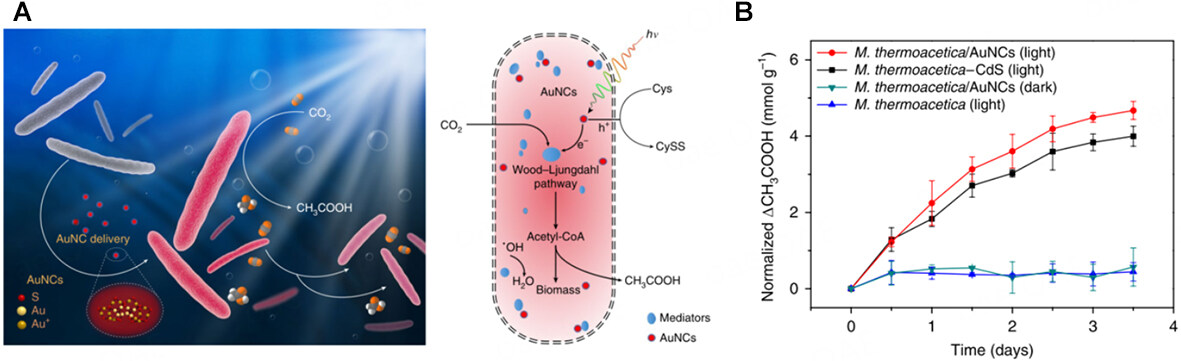
Noble MNCs exhibit excellent biocompatibility, minimizing interference with the inherent functions of living organisms. This advantage allows noble MNCs to be used in the biological field. To link pre-assembled biosynthetic pathways with inorganic light absorbers, a photosynthetic biohybrid system (PBS) was developed. Both the high light-harvesting efficiency of solid-state semiconductors and the superior catalytic performance of whole-cell microorganisms are inherited by this strategy[108]. For instance,
Figure 17. (A) Schematic diagram of the M. thermoacetica/Au NCs hybrid system. (B) photosynthesis behavior of different systems[109]. Copyright 2018, Springer Nature.
To summarize, compared to noble MNCs-based catalysts, nonnoble MNCs-based catalysts have a lower price, greater availability, a more comprehensive selection of materials, and better stability. The disadvantage is that the catalytic effect of the PCR process is inferior to that of noble MNCs. Furthermore, noble MNCs-based catalysts exhibit better biocompatibility. However, the scarcity of precious metals causes them to be expensive. The high specific surface area of noble MNCs-based catalysts can cause aggregation. Maintaining the long-term stability of the composites under in situ light conditions is challenging, a bottleneck in developing noble MNCs-based catalysts for practical applications. Thus, adopting strategies to prevent cluster aggregation on the carrier (such as Z/S-scheme heterojunction or MOF structure) is essential for their photocatalytic performance.
SUMMARY AND PROSPECTS
Artificial carbon fixation has stimulated the development of PCR as a promising technology. MNCs are a new potential photocatalyst with unique physical and chemical properties. To improve PCR performance, various advanced techniques have been widely explored. Due to the fast progress of this field, a summary of recent studies is essential for both new and experienced researchers in related fields. In this review, we summarize the recent advances in MNCs-based catalysts for PCR. We first introduce the synthesis of MNCs. Then, we explain the mechanism of photocatalysis based on MNCs-based catalysts. Finally, in the section on advanced MNCs-based catalysts for PCR, we classify MNCs into nonnoble MNCs and noble MNCs and summarize their role as catalysts in enhancing PCR performance. In the future, it is believed that there will be more strategies to optimize the design of MNCs-based catalysts for PCR. Therefore, we want to emphasize the outlook for catalysts based on MNCs:
(1) The majority of current research is devoted to the development of transition MNCs. However, other types of NCs, such as alloy NCs, also merit investigation and consideration.
(2) The selectivity of CO2 reduction by MNCs-based catalysts used to enhance the PCR process is still not good, and most systems can only reduce CO2 to C1 products such as CO. Therefore, how to improve the selectivity of PCR through material and system design is an important challenge at present.
(3) In the PCR process, MNCs are mostly used as co-catalysts/photosensitizers for semiconductor nanomaterials. It is hoped that in the future, there will be MNCs that can be used directly in PCR with excellent performance.
(4) The PCR efficiencies are still lower than commercial targets, and a firm understanding of how these rich structures of MNCs affect catalytic performance has yet to be fully achieved. The reaction pathways at the atomic level have yet to be determined. Therefore, further research should be dedicated to enhancing the activity of PCR processes and monitoring the structure of MNCs during reactions to further establish the precise correlation between structure and catalytic performance.
DECLARATIONS
Authors’ contributionsConceptualization, investigation, writing-original draft: Li H
Writing-review & editing: Luo H, Du H
Writing-review & editing, supervision, and funding acquisition: Wang H, Zhu W, Zhou Y
Availability of data and materialsNot applicable.
Financial support and sponsorshipThis work was supported by the National Natural Science Foundation of China (22176086), the Natural Science Foundation of Jiangsu Province (BK20210189), State Key Laboratory of Pollution Control and Resource Reuse (PCRR-ZZ-202106), the Fundamental Research Funds for the Central Universities (021114380183, 021114380189, 021114380199), the Research Funds from the Nanjing Science and Technology Innovation Project for Chinese Scholars Studying Abroad (13006003), the Research Funds from Frontiers Science Center for Critical Earth Material Cycling of Nanjing University, and Research Funds for Jiangsu Distinguished Professor. Y.Z. would like to acknowledge the support from the National Natural Science Foundation of China (22276100), Natural Science Foundation of Jiangsu Province (SBK2022044384), Key Laboratory for Organic Electronics & Information Displays (GZR2022010010), Nanjing Science and Technology Innovation Project for Chinese Scholars Studying Abroad (NJKCZYZZ2022-01), Research Fund for Jiangsu Distinguished Professor (RK030STP22001), and the Research startup fund of NJUPT (NY221006).
Conflicts of interestAll authors declared that there are no conflicts of interest.
Ethical approval and consent to participateNot applicable.
Consent for publicationNot applicable.
Copyright© The Author(s) 2023.
REFERENCES
1. Arneth A, Sitch S, Pongratz J, et al. Historical carbon dioxide emissions caused by land-use changes are possibly larger than assumed. Nat Geosci 2017;10:79-84.
2. Liu LX, Fu J, Jiang LP, Zhang JR, Zhu W, Lin Y. Highly efficient photoelectrochemical reduction of CO2 at low applied voltage using 3D Co-Pi/BiVO4/SnO2 nanosheet array photoanodes. ACS Appl Mater Interfaces 2019;11:26024-31.
4. Liu J, Fu J, Zhou Y, Zhu W, Jiang LP, Lin Y. Controlled synthesis of EDTA-modified porous hollow copper microspheres for high-efficiency conversion of CO2 to multicarbon products. Nano Lett 2020;20:4823-8.
5. Liu J, Cai Y, Song R, et al. Recent progress on single-atom catalysts for CO2 electroreduction. Mater Today 2021;48:95-114.
6. Salemdeeb R, Saint R, Clark W, Lenaghan M, Pratt K, Millar F. A pragmatic and industry-oriented framework for data quality assessment of environmental footprint tools. Resour Environ Sustain 2021;3:100019.
7. Dou X, Wang Y, Ciais P, et al. Near-real-time global gridded daily CO2 emissions. Innovation 2022;3:100182.
8. Du H, Liu LX, Li P, et al. Enriching reaction intermediates in multishell structured copper catalysts for boosted propanol electrosynthesis from carbon monoxide. ACS Nano 2023;17:8663-70.
9. Zhao Q, Yu P, Mahendran R, et al. Global climate change and human health: pathways and possible solutions. Eco-Environ Health 2022;1:53-62.
10. Fu J, Li P, Lin Y, et al. Fight for carbon neutrality with state-of-the-art negative carbon emission technologies. Eco-Environ Health 2022;1:259-79.
11. Li K, Cai Y, Yang X, et al. H2S Involved photocatalytic system: a novel syngas production strategy by boosting the photoreduction of CO2 while recovering hydrogen from the environmental toxicant. Adv Funct Mater 2022;32:2113002.
12. Yang X, Li K, Wang G, et al. 2D Catalysts for CO2 photoreduction: discussing structure efficiency strategies and prospects for scaled production based on current progress. Chemistry 2022;28:e202201881.
13. Ran J, Jaroniec M, Qiao SZ. Cocatalysts in semiconductor-based photocatalytic CO2 reduction: achievements, challenges, and opportunities. Adv Mater 2018;30:1704649.
14. Fu J, Jiang K, Qiu X, Yu J, Liu M. Product selectivity of photocatalytic CO2 reduction reactions. Mater Today 2020;32:222-43.
16. Tian J, Zhong K, Zhu X, et al. Highly exposed active sites of Au nanoclusters for photocatalytic CO2 reduction. Chem Eng J 2023;451:138392.
17. Yang J, Yang Z, Yang K, et al. Indium-based ternary metal sulfide for photocatalytic CO2 reduction application. Chin J Catal 2023;44:67-95.
18. Zhu L, Hu F, Sun B, Gu S, Gao T, Zhou G. Recent advances on multivariate MOFs for photocatalytic CO2 reduction and H2 evolution. Adv Sustain Syst 2023;7:2200394.
19. Zhu Z, Xuan Y, Liu X, Zhu Q. Revealing the stochastic kinetics evolution of photocatalytic CO2 reduction. Nanoscale 2023;15:730-41.
20. Zuo Q, Cui R, Wang L, et al. High-loading single cobalt atoms on ultrathin MOF nanosheets for efficient photocatalytic CO2 reduction. Sci China Chem 2023;66:570-7.
21. Liu H, Zhu Y, Ma J, Zhang Z, Hu W. Recent advances in atomic-level engineering of nanostructured catalysts for electrochemical CO2 reduction. Adv Funct Mater 2020;30:1910534.
22. He L, Yuan J, Xia N, et al. Kernel tuning and nonuniform influence on optical and electrochemical gaps of bimetal nanoclusters. J Am Chem Soc 2018;140:3487-90.
23. Bootharaju MS, Baek W, Lee S, Chang H, Kim J, Hyeon T. Magic-sized stoichiometric II-VI nanoclusters. Small 2021;17:e2002067.
24. Busatto S, de Mello Donega C. Magic-size semiconductor nanostructures: where does the magic come from? ACS Mater Au 2022;2:237-49.
25. Wang Y, Zhou Y, Zhang Y, Buhro WE. Magic-size II-VI nanoclusters as synthons for flat colloidal nanocrystals. Inorg Chem 2015;54:1165-77.
26. Kurashige W, Kumazawa R, Ishii D, et al. Au25-loaded BaLa4Ti4O15 water-splitting photocatalyst with enhanced activity and durability produced using new chromium oxide shell formation method. J Phys Chem C 2018;122:13669-81.
27. Gautam A, Gore PM, Kandasubramanian B. Nanocluster materials in photosynthetic machines. Chem Eng J 2020;385:123951.
28. Nitopi S, Bertheussen E, Scott SB, et al. Progress and perspectives of electrochemical CO2 reduction on copper in aqueous electrolyte. Chem Rev 2019;119:7610-72.
29. Shoji S, Yin G, Nishikawa M, Atarashi D, Sakai E, Miyauchi M. Photocatalytic reduction of CO2 by CuO nanocluster loaded SrTiO3 nanorod thin film. Chem Phys Lett 2016;658:309-14.
30. Gao Y, Sun L, Bian J, Zhang Z, Li Z, Jing L. Accelerated charge transfer of g-C3N4/BiVO4 Z-scheme 2D heterojunctions by controllably introducing phosphate bridges and Ag nanocluster co-catalysts for selective CO2 photoreduction to CO. Appl Surf Sci 2023;610:155360.
31. Bo Y, Du P, Li H, et al. Bridging Au nanoclusters with ultrathin LDH nanosheets via ligands for enhanced charge transfer in photocatalytic CO2 reduction. Appl Catal B Environ 2023;330:122667.
32. Chen J, Zhang QF, Bonaccorso TA, Williard PG, Wang LS. Controlling gold nanoclusters by diphospine ligands. J Am Chem Soc 2014;136:92-5.
33. Zhu Q, Huang X, Zeng Y, et al. Controllable synthesis and electrocatalytic applications of atomically precise gold nanoclusters. Nanoscale Adv 2021;3:6330-41.
34. Liu L, Corma A. Metal Catalysts for heterogeneous catalysis: from single atoms to nanoclusters and nanoparticles. Chem Rev 2018;118:4981-5079.
35. Chakraborty I, Pradeep T. Atomically precise clusters of noble metals: emerging link between atoms and nanoparticles. Chem Rev 2017;117:8208-71.
36. Lu H, Chen B, Li Y, et al. Benzyl-rich ligand engineering of the photostability of atomically precise gold nanoclusters. Chem Commun 2022;58:2395-8.
37. Fang J, Zhang B, Yao Q, Yang Y, Xie J, Yan N. Recent advances in the synthesis and catalytic applications of ligand-protected, atomically precise metal nanoclusters. Coord Chem Rev 2016;322:1-29.
38. Chai OJH, Liu Z, Chen T, Xie J. Engineering ultrasmall metal nanoclusters for photocatalytic and electrocatalytic applications. Nanoscale 2019;11:20437-48.
39. Sun Y, Cai X, Hu W, Liu X, Zhu Y. Electrocatalytic and photocatalytic applications of atomically precise gold-based nanoclusters. Sci China Chem 2021;64:1065-75.
40. Wu J, Xia W, Lan M, et al. Artificial photosynthetic assemblies constructed by the self-assembly of synthetic building blocks for enhanced photocatalytic hydrogen evolution. J Mater Chem A 2020;8:21690-9.
41. Yao Q, Chen T, Yuan X, Xie J. Toward total synthesis of thiolate-protected metal nanoclusters. ACC Chem Res 2018;51:1338-48.
42. Luo Z, Nachammai V, Zhang B, et al. Toward understanding the growth mechanism: tracing all stable intermediate species from reduction of Au(I)-thiolate complexes to evolution of Au25 nanoclusters. J Am Chem Soc 2014;136:10577-80.
43. Yao Q, Yuan X, Fung V, et al. Understanding seed-mediated growth of gold nanoclusters at molecular level. Nat Commun 2017;8:927.
44. Wang S, Li Q, Kang X, Zhu M. Customizing the structure, composition, and properties of alloy nanoclusters by metal exchange. ACC Chem Res 2018;51:2784-92.
45. Li Y, Zhou M, Jin R. Programmable metal nanoclusters with atomic precision. Adv Mater 2021;33:e2006591.
46. Li G, Jin R. Atomically precise gold nanoclusters as new model catalysts. ACC Chem Res 2013;46:1749-58.
47. Zhou M, Higaki T, Li Y, et al. Three-stage evolution from nonscalable to scalable optical properties of thiolate-protected gold nanoclusters. J Am Chem Soc 2019;141:19754-64.
48. Pan H, Heagy MD. Photons to formate-a review on photocatalytic reduction of CO2 to formic acid. Nanomaterials 2020;10:2422.
49. Linsebigler AL, Lu G, Yates JT. Photocatalysis on TiO2 surfaces: principles, mechanisms, and selected results. Chem Rev 1995;95:735-58.
50. Habisreutinger SN, Schmidt-mende L, Stolarczyk JK. Photokatalytische reduktion von CO2 an TiO2 und anderen halbleitern. Angew Chem Int Ed 2013;125:7516-57.
51. Yan J, Teo BK, Zheng N. Surface chemistry of atomically precise coinage-metal nanoclusters: from structural control to surface reactivity and catalysis. ACC Chem Res 2018;51:3084-93.
52. Hou B, Kim B, Lee HKH, et al. Multiphoton absorption stimulated metal chalcogenide quantum dot solar cells under ambient and concentrated irradiance. Adv Funct Mater 2020;30:2004563.
53. Guo K, Zhu X, Peng L, et al. Boosting photocatalytic CO2 reduction over a covalent organic framework decorated with ruthenium nanoparticles. Chem Eng J 2021;405:127011.
54. Kuhl KP, Cave ER, Abram DN, Jaramillo TF. New insights into the electrochemical reduction of carbon dioxide on metallic copper surfaces. Energy Environ Sci 2012;5:7050.
55. Zhou M, Wang S, Yang P, Huang C, Wang X. Boron carbon nitride semiconductors decorated with CdS nanoparticles for photocatalytic reduction of CO2. ACS Catal 2018;8:4928-36.
56. Nguyen D, Nguyen C, Do T. Rational one-step synthesis of cobalt clusters embedded-graphitic carbon nitrides for the efficient photocatalytic CO2 reduction under ambient conditions. J Catal 2020;392:88-96.
57. Hansen HA, Varley JB, Peterson AA, Nørskov JK. Understanding trends in the electrocatalytic activity of metals and enzymes for CO2 reduction to CO. J Phys Chem Lett 2013;4:388-92.
58. Rosen BA, Salehi-Khojin A, Thorson MR, et al. Ionic liquid-mediated selective conversion of CO2 to CO at low overpotentials. Science 2011;334:643-4.
59. Palencia C, Yu K, Boldt K. The future of colloidal semiconductor magic-size clusters. ACS Nano 2020;14:1227-35.
60. Chang X, Wang T, Gong J. CO2 photo-reduction: insights into CO2 activation and reaction on surfaces of photocatalysts. Energy Environ Sci 2016;9:2177-96.
61. Peng S, Zeng X, Li Y. Titanate nanotube modified with different nickel precursors for enhanced Eosin Y-sensitized photocatalytic hydrogen evolution. Int J Hydrog Energy 2015;40:6038-49.
62. Zhang W, Li Y, Zeng X, Peng S. Synergetic effect of metal nickel and graphene as a cocatalyst for enhanced photocatalytic hydrogen evolution via dye sensitization. Sci Rep 2015;5:10589.
63. Li Y, Xiang Y, Peng S, Wang X, Zhou L. Modification of Zr-doped titania nanotube arrays by urea pyrolysis for enhanced visible-light photoelectrochemical H2 generation. Electrochim Acta 2013;87:794-800.
64. Yin G, Nishikawa M, Nosaka Y, et al. Photocatalytic carbon dioxide reduction by copper oxide nanocluster-grafted niobate nanosheets. ACS Nano 2015;9:2111-9.
65. Park D, Jeong Y, Lee J, Lee J, Moon S. Interfacial charge-transfer loss in dye-sensitized solar cells. J Phys Chem C 2013;117:2734-9.
66. Irie H, Miura S, Kamiya K, Hashimoto K. Efficient visible light-sensitive photocatalysts: Grafting Cu(II) ions onto TiO2 and WO3 photocatalysts. Chem Phys Lett 2008;457:202-5.
67. Irie H, Kamiya K, Shibanuma T, et al. Visible light-sensitive Cu(II)-grafted TiO2 photocatalysts: activities and X-ray absorption fine structure analyses. J Phys Chem C 2009;113:10761-6.
68. Yu H, Irie H, Shimodaira Y, et al. An efficient visible-light-sensitive Fe(III)-grafted TiO2 photocatalyst. J Phys Chem C 2010;114:16481-7.
69. Liu M, Qiu X, Hashimoto K, Miyauchi M. Cu(II) nanocluster-grafted, Nb-doped TiO2 as an efficient visible-light-sensitive photocatalyst based on energy-level matching between surface and bulk states. J Mater Chem A 2014;2:13571-9.
70. Miyauchi M, Irie H, Liu M, et al. Visible-light-sensitive photocatalysts: nanocluster-grafted titanium dioxide for indoor environmental remediation. J Phys Chem Lett 2016;7:75-84.
71. Kong L, Wang C, Wan F, Zheng H, Zhang X. Synergistic effect of surface self-doping and Fe species-grafting for enhanced photocatalytic activity of TiO2 under visible-light. Appl Surf Sci 2017;396:26-35.
72. Ji Y, Luo Y. New Mechanism for photocatalytic reduction of CO2 on the anatase TiO2 (101) surface: the essential role of oxygen vacancy. J Am Chem Soc 2016;138:15896-902.
73. Nolan M, Iwaszuk A, Gray KA. Localization of photoexcited electrons and holes on low coordinated Ti and O sites in free and supported TiO2 nanoclusters. J Phys Chem C 2014;118:27890-900.
74. Hurum D, Agrios A, Crist S, Gray K, Rajh T, Thurnauer M. Probing reaction mechanisms in mixed phase TiO2 by EPR. J Electron Spectros Relat Phenomena 2006;150:155-63.
75. Li G, Gray KA. The solid-solid interface: explaining the high and unique photocatalytic reactivity of TiO2-based nanocomposite materials. Chem Phys 2007;339:173-87.
77. Liu M, Qiu X, Miyauchi M, Hashimoto K. Energy-level matching of Fe(III) ions grafted at surface and doped in bulk for efficient visible-light photocatalysts. J Am Chem Soc 2013;135:10064-72.
78. Liu M, Sunada K, Hashimoto K, Miyauchi M. Visible-light sensitive Cu(II)-TiO2 with sustained anti-viral activity for efficient indoor environmental remediation. J Mater Chem A 2015;3:17312-9.
79. Liu M, Inde R, Nishikawa M, et al. Enhanced photoactivity with nanocluster-grafted titanium dioxide photocatalysts. ACS Nano 2014;8:7229-38.
80. Cheng L, Li B, Yin H, Fan J, Xiang Q. Cu clusters immobilized on Cd-defective cadmium sulfide nano-rods towards photocatalytic CO2 reduction. J Mater Sci Technol 2022;118:54-63.
81. Billo T, Fu FY, Raghunath P, et al. Ni-nanocluster modified black TiO2 with dual active sites for selective photocatalytic CO2 reduction. Small 2018;14:1702928.
82. Li Y, Wang C, Song M, Li D, Zhang X, Liu Y. TiO2-x/CoOx photocatalyst sparkles in photothermocatalytic reduction of CO2 with
83. Hou T, Luo N, Cui Y, et al. Selective reduction of CO2 to CO under visible light by controlling coordination structures of CeOx-S/ZnIn2S4 hybrid catalysts. Appl Catal B Environ 2019;245:262-70.
84. Mrowetz M, Villa A, Prati L, Selli E. Effects of Au nanoparticles on TiO2 in the photocatalytic degradation of an azo dye. Gold Bull 2007;40:154-60.
85. Yadav A, Li Y, Liao TW, et al. Enhanced methanol electro-oxidation activity of nanoclustered gold. Small 2021;17:e2004541.
86. Liao TW, Verbruggen SW, Claes N, et al. TiO2 films modified with Au nanoclusters as self-cleaning surfaces under visible light. Nanomaterials 2018;8:30.
87. Li Y, Yang Y, Chen G, Fan J, Xiang Q. Au cluster anchored on TiO2/Ti3C2 hybrid composites for efficient photocatalytic CO2 reduction. Rare Met 2022;41:3045-59.
88. Xiao FX, Zeng Z, Hsu SH, Hung SF, Chen HM, Liu B. Light-induced
89. Liu S, Xu YJ. Photo-induced transformation process at gold clusters-semiconductor interface: implications for the complexity of gold clusters-based photocatalysis. Sci Rep 2016;6:22742.
90. Zhou P, Yu J, Jaroniec M. All-solid-state Z-scheme photocatalytic systems. Adv Mater 2014;26:4920-35.
91. Bard AJ. Photoelectrochemistry and heterogeneous photo-catalysis at semiconductors. J Photochem 1979;10:59-75.
92. Li H, Tu W, Zhou Y, Zou Z. Z-scheme photocatalytic systems for promoting photocatalytic performance: recent progress and future challenges. Adv Sci 2016;3:1500389.
93. Maeda K. Z-scheme water splitting using two different semiconductor photocatalysts. ACS Catal 2013;3:1486-503.
94. Xu Q, Zhang L, Cheng B, Fan J, Yu J. S-scheme heterojunction photocatalyst. Chem 2020;6:1543-59.
95. Deng Y, Zhang Z, Du P, et al. Embedding ultrasmall Au clusters into the pores of a covalent organic framework for enhanced photostability and photocatalytic performance. Angew Chem Int Ed 2020;132:6138-45.
96. Xu Q, Wageh S, Al-ghamdi AA, Li X. Design principle of S-scheme heterojunction photocatalyst. J Mater Sci Technol 2022;124:171-3.
97. Li X, Xiong J, Gao X, et al. Novel BP/BiOBr S-scheme nano-heterojunction for enhanced visible-light photocatalytic tetracycline removal and oxygen evolution activity. J Hazard Mater 2020;387:121690.
98. Xia P, Cao S, Zhu B, et al. Designing a 0D/2D S-scheme heterojunction over polymeric carbon nitride for visible-light photocatalytic inactivation of bacteria. Angew Chem Int Ed 2020;59:5218-25.
99. Xu F, Meng K, Cheng B, Wang S, Xu J, Yu J. Unique S-scheme heterojunctions in self-assembled TiO2/CsPbBr3 hybrids for CO2 photoreduction. Nat Commun 2020;11:4613.
100. Wageh S, A. Al-ghamdi A, Liu L. S-scheme heterojunction photocatalyst for CO2 photoreduction. Acta Physico-Chimica Sinica 2021;37:2010024.
102. Ke X, Zhang J, Dai K, Fan K, Liang C. Integrated S-scheme heterojunction of amine-functionalized 1D CdSe nanorods anchoring on ultrathin 2D SnNb2O6 Nanosheets for robust solar-driven CO2 conversion. Solar RRL 2021;5:2000805.
104. Long JR, Yaghi OM. The pervasive chemistry of metal-organic frameworks. Chem Soc Rev 2009;38:1213-4.
105. Bernales V, Ortuño MA, Truhlar DG, Cramer CJ, Gagliardi L. Computational design of functionalized metal-organic framework nodes for catalysis. ACS Cent Sci 2018;4:5-19.
106. Choudhuri I, Truhlar DG. Photogenerated charge separation in a CdSe nanocluster encapsulated in a metal-organic framework for improved photocatalysis. J Phys Chem C 2020;124:8504-13.
107. Jiang Y, Yu Y, Zhang X, et al. N-heterocyclic carbene-stabilized ultrasmall gold nanoclusters in a metal-organic framework for photocatalytic CO2 reduction. Angew Chem Int Ed 2021;60:17388-93.
108. Sakimoto KK, Kornienko N, Yang P. Cyborgian material design for solar fuel production: the emerging photosynthetic biohybrid systems. ACC Chem Res 2017;50:476-81.
Cite This Article
Export citation file: BibTeX | RIS
OAE Style
Li H, Du H, Luo H, Wang H, Zhu W, Zhou Y. Recent developments in metal nanocluster-based catalysts for improving photocatalytic CO2 reduction performance. Microstructures 2023;3:2023024. http://dx.doi.org/10.20517/microstructures.2023.09
AMA Style
Li H, Du H, Luo H, Wang H, Zhu W, Zhou Y. Recent developments in metal nanocluster-based catalysts for improving photocatalytic CO2 reduction performance. Microstructures. 2023; 3(3): 2023024. http://dx.doi.org/10.20517/microstructures.2023.09
Chicago/Turabian Style
Li, Huan, Huitong Du, Huanhuan Luo, Hua Wang, Wenlei Zhu, Yang Zhou. 2023. "Recent developments in metal nanocluster-based catalysts for improving photocatalytic CO2 reduction performance" Microstructures. 3, no.3: 2023024. http://dx.doi.org/10.20517/microstructures.2023.09
ACS Style
Li, H.; Du H.; Luo H.; Wang H.; Zhu W.; Zhou Y. Recent developments in metal nanocluster-based catalysts for improving photocatalytic CO2 reduction performance. Microstructures. 2023, 3, 2023024. http://dx.doi.org/10.20517/microstructures.2023.09
About This Article
Copyright
Data & Comments
Data
 Cite This Article 30 clicks
Cite This Article 30 clicks













Comments
Comments must be written in English. Spam, offensive content, impersonation, and private information will not be permitted. If any comment is reported and identified as inappropriate content by OAE staff, the comment will be removed without notice. If you have any queries or need any help, please contact us at support@oaepublish.com.