The treatment of nerve defects
Abstract
Among the many challenges that the hand surgeon has to face in his daily work, nerve defects of the hand represent one of the hardest; unsatisfactory results in their treatment may cause severe limitations for the patient’s social and working life. Many advances have been made over the years in the treatment of such conditions, and at the current state, surgical treatment can achieve satisfactory results. This article aims to review the current concepts in hand innervation and nerve pathophysiology and to describe traditional and novel surgical techniques currently employed to correct these defects.
Keywords
INTRODUCTION
Amid hand injuries, peripheral nerve lesions are a common issue; among surgically treated nerve injuries, digital nerve repair is the most frequent procedure[1]. Various mechanisms can cause these types of lesions, although direct mechanical trauma, possibly associated with other soft and bone tissue injuries, is the most relevant[2]. In 2020, a study regarding the incidence of nerve injuries after extremity trauma in the United States showed that the hands were one of the most frequent sites of injuries[3]; this can be explained by the fact that in case of injury, the hands and the arms and forearms as well are out-stretched frequently as a protective instinct, often involving the dominant hand[4]. Peripheral Nerve Injuries (PNIs) may result in poor long-term clinical outcomes in cases of suboptimal sensory recovery, chronic neuropathic pain, and impaired motor function, thus afflicting life quality[5]. Wide variability in clinical presentation, proposed treatments and outcomes makes hand PNI a potential challenge for hand surgeons; to achieve satisfactory results, it is important to have a clear knowledge of peripheral nerves anatomy at the wrist and hand level, of the physiopathological mechanisms occurring after trauma, of the strategies that have been developed to treat them and the proper timing of management.
ANATOMY
Sensory and motor innervation of the wrist and hand is guaranteed by three nerves: median, ulnar, and radial nerves.
The Median Nerve (MN), just before entering the carpal tunnel, releases the palmar cutaneous branch of the median nerve[6], which supplies afferent fibers to the palmar surface of the hand as a constant structure, occasionally mentioned as absent in literature[7]; this sensory branch common spring from the radial side of the MN at an average distance of 4.1cm to 8.4 cm proximal to the volar wrist crease, with a mean length of the nerve spanning from 2cm to 15cm[8-10]; the palmar cutaneous branch of the median nerve has been shown to possess in several studies a high anatomical variability of both pathway and branching which are complicating factors in predicting its location during surgical procedures[11].
Exiting the carpal tunnel, MN splits into a medial ad a lateral branch. The first and the second palmar common digital nerves originate from the medial branch; they provide motor fibers for the second lumbrical muscle and sensory fibers for the distal palm, the third finger, half of the index finger and, usually, half of the ring finger; from the lateral branch, instead, arise the thenar motor branch and the first three palmar proper digital nerves innervating the first lumbrical muscle and supplying sensory innervation to the radial half of the index finger and the thumb; possible variants of the anatomical pathway of thenar motor branch have been studied, but in the most frequent cases it arises from the radial side of MN, distal to the transverse carpal ligament[12].
In the ulnar-palmar wrist region, the Ulnar Nerve (UN), emerging from underneath the flexor carpi ulnaris tendon, releases the dorsal branch of the UN nerve. This branch directs dorsally and carries sensitive fibers to the dorsal-ulnar region of the hand[13] and supplies sensitive innervation of the hypothenar eminence thanks to small branches[12]; subsequently the UN enters Guyon’s canal, where, approximately at the level of the distal edge of the pisiform, splits into a superficial and a deep branches: the superficial branch divides further into the proper digital nerve of the little finger, the common digital nerve of the fourth web space, a cutaneous branch for the palmar surface and a motor branch for the palmaris brevis muscle; the deep branch is purely motor and travels deep and ulnar in relationship to the superficial branch, commonly giving off firstly, inside or outside the canal, fiber for the abductor digiti minimi muscle, it then curves radially around the hook of the hamate, along with the deep branch of the ulnar artery, providing motor innervation for the hypothenar muscles, adductor pollicis and flexor pollicis brevis[14,15]. Lumbrical and interosseous muscles also possess variable innervation: the first and second lumbrical usually receive motor innervation from MN branches; instead, the third lumbrical gets both UN and MN innervation up to 64% of the cases [16]; interosseous muscles innervation is often provided by the deep branch of the UN, but it has been demonstrated, during autopsy studies, that in up to 3% of cases, the first dorsal interosseus is innervated by MN[12].
To complicate the scenario, many anastomoses between the UN and MN can occur, altering pathways of innervation. The Martin-Gruber anastomosis occurs in the forearm between the anterior interosseous nerve (or the MN itself) and the UN; it carries motor fibers from the proximal MN to the distal UN, serving principally the first dorsal interosseous muscle and, possibly, the hypothenar muscles too[12,17]. In rare cases, this communication can take place even in the opposite direction, in which case it is named “Marinacci anastomosis”[12-14,17-18]. In the hand, the anastomosis between the thenar motor branch and the deep branch of the UN is called Thenar Ansa, or Riche-Cannieu anastomosis; in this case, the involvement of motor and sensitive fibers can lead to a hand solely supplied by the UN[17,19].
The Berrettini anastomosis is the most frequent of the anomalies, and it is a purely sensory anatomical alteration between the third and the fourth common digital nerve, causing alterations in the sensibility of the middle and ring fingers[12,17].
The third nerve innervating the wrist and the hand is the radial nerve (RN) through its superficial branch; it is entirely sensory and, at the radial styloid, divides into several distal sensory branches which innervate the radial region of the dorsum of the hand as well as the dorsal side of the thumb, index, middle and radial half of ring fingers[20].
PHYSIOPATHOLOGICAL MECHANISMS
PNIs are traditionally classified by Seddon[21] and Sunderland[22] classifications.
In 1943, Seddon first proposed a division into three groups of PNIs: neurapraxia, axonotmesis, and neurotmesis; secondly, Sunderland expanded Seddon’s classification into five grades based on the involvement of the nerve’s structures.
Neuroapraxia, corresponding to Sunderland grade 1, means a compression or traction injury damaging Schwann cell sheath and leading to focal demyelination but without structural nerve interruption and degeneration; this provokes a conduction block with transient loss of function until remyelination occurs, usually in a period ranging from hours to some weeks, with full recovery potential; at this stage, an advancing Tinel sign is not appreciated.
When axonotmesis occurs, the damage spreads to the axon with variable involvement of the internal nerve structures. In this scenario, a process of degeneration and regeneration, called Wallerian degeneration, takes place within the first 48–72 hours, serving a favorable environment for axonal regrowth thanks to the upregulation of several trophic factors served by the surrounding proliferating Schwann cells; the regrowth can proceeds, in the right conditions, at a speed of up to 2mm/day but, since this process decreases over time, any surgical interventions should be planned promptly[23,24].
Progressive disruption of the internal nerve structures (endoneurium, perineurium, and epineurium) increases the rate of scarring and intraneural fibrosis, altering perineural vascularization and disturbing axonal regeneration and nerve recovery[23]. Considering the prognostic relevance of this factor, Sunderland further subdivided axonotmesis into three grades.
In grade 2, only the axon and its myelin sheath are disrupted without harm to connective structures: denervation with loss of motor and sensory function occurs, but since the endoneurium is not involved, poor scar tissue is produced; thus, complete nerve regeneration with optimal recovery is expected without surgical intervention[24]; it is the first stage showing an advancing Tinel sign, meaning the nerve regeneration is progressing[25].
In grade 3, the injury reaches the endoneurium: more scarring and disorganization in nerve regeneration are expected, although a slow advancing Tinel sign could be recognized; under these circumstances, full recovery is hardly achievable, and in severe cases, surgical excision and nerve reconstruction may be required[25,26]. In grade 4, epineurium is the only structure remaining intact; Tinel sign is present at injury level, but it does not advance due to the huge amount of fibrosis. Function restoration is such impaired that surgical intervention is mostly required[24-27]. Neurotmesis, corresponding to a grade 5, indicates a complete discontinuity of the nerve ends; thus, surgical intervention is mandatory to achieve functional recovery[24]. Lastly, Mackinnon[28] introduced a grade 6 injury in which more traumatic mechanisms are involved; in these “mixed injuries”, a combination of findings mentioned above can co-exist.
TREATMENTS
End-to-end neurorrhaphy
Alignment and coaptation of nerve endings with epineural microsutures are the gold standard treatment for higher-grade axonotmesis and neurotmesis injuries[26,27]. However, it is essential to achieve tension-free coaptation, as increased tension has been shown to negatively impact blood flow, impairing the healing processes of the nerve; trimming the proximal and distal stumps removes nonviable tissues that might interfere with the healing process, then nerve endings are mobilized to overcome elastic forces and achieve a tension-free repair. As tension-free coaptation might be subjective, an idea of the proper amount of tension can be observed by positioning only one stitch between the nerve’s ends: if the stitch can withstand the retracting tension between the two nerve ends, it is possible to carry on the coaptation.
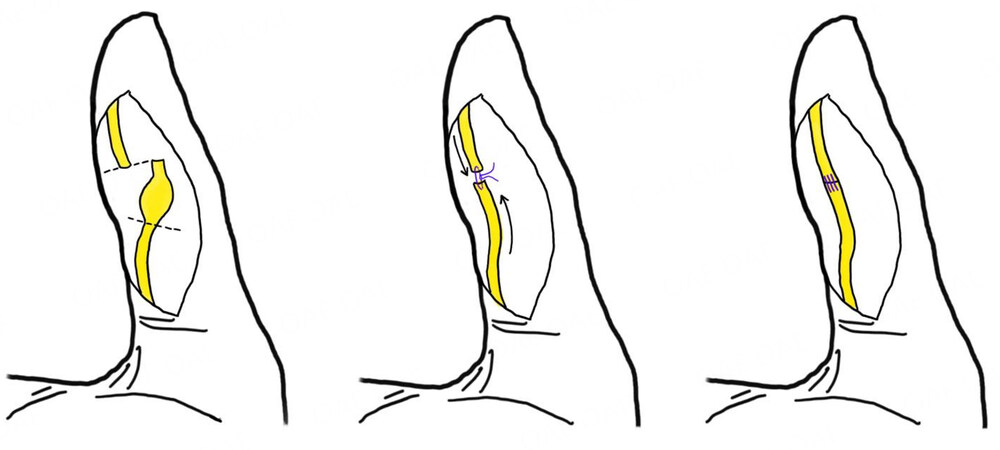
Finally, the nerve defect is closed with microsurgical sutures, usually 8-0 to 10-0, to minimize the formation of foreign body scar tissue, avoiding excessive tension[24,28] [Figure 1]. In case of more proximal lesions at wrist level, an interfascicular suture is suggested; the outer epineurium is pulled back to expose individual fascicles which are approximated in an end-to-end fashion with a single 10-0 suture placed through the perineurium. In order to obtain a better nerve repair, the fascicular pattern can be better identified by observing the epineural blood vessels.
Figure 1. Digital neuroma following sharp-force trauma; neuroma has been excised, trimming the proximal and distal stumps, then an end-to-end tension-free neurorrhaphy has been performed.
Commercial fibrin glue in nerve reconstructions is commonly used as it provides an adhesive layer shielding the defect and, possibly, has positive effects upon inflammatory and fibrotic response; despite its employ alone, it is still the object of dispute, the addition of fibrin glue seems to provide a useful aid to neurorrhaphy[24,26,29]. In a study of Sallam et al., motor and sensory recovery of autologous fibrin glue application was compared with a standard micro-suturing technique for nerve defects at the forearm and wrist levels: the authors reported that the use of fibrin glue was as effective as microsuturing in regaining motor and sensory functions and associated with shorter operative time[30].
Following surgery, a period of immobilization with plaster for three weeks is recommended to limit movement and tension at the coaptation site in order to protect the suture, even if some authors suggest an immobilization after isolated digital nerve reconstruction for 10 days or even shorter[31].
Nerve reconstruction techniques
Sometimes, the nerve’s defect could be so long to prevent suturing without excessive tension or to restrain ends coaptation; in these cases, a nerve reconstruction is indicated. Reversed autologous nerve grafts are the best option since they offer a reliable scaffold full of stimulating elements, such as Schwann cell basal laminae and neurotrophic factors[26,27]. Nerve ends are prepared in the same fashion as to perform an end-to-end neurorrhaphy; after that, the length of the defect is measured to calculate how much of the donor nerve will be taken, estimating an extra percentage of 10% to 15% due to nerve graft’s shrinkage[24,32]. Common donor sites for these procedures are the sural, saphenous, sensory branch of the RN, medial or lateral antebrachial cutaneous nerves, and the posterior interosseous nerve; in particular, the last two nerves are the most suitable in the reconstruction of common and proper digital nerves because their loss is poorly relevant for the patient[24,33-35].
Despite being the gold standard procedure, several disadvantages have been reported in autologous nerve grafts employ, such as scarcity of donor material, increased surgical time for harvesting the autograft, graft and donor-site morbidity, loss of nerve function and potential neuroma formation in the donor site, and possible mismatch between donor and recipient nerves[26,27,36].
Vascularized nerve grafts in nerve reconstruction are employed in brachial plexus disorders, and their use in the distal wrist and hand districts is uncommon. As a result, new solutions have been developed as alternatives to autologous graft; commercial options consist in bridging the defect through biodegradable nerve conduits composed of polymer-based materials or processed decellularized human nerve allografts. Biodegradable nerve conduits protect the growing axons through a semipermeable cavity and create a microenvironment rich in chemical factors that upregulate nerve regeneration; decellularized human nerve allografts provide an internal microstructure and extracellular matrix molecules of the native nerve tissue[37]. Nerve conduits are easily applied in the nerve gaps by positioning sutures through the conduit and the epineurium in a “U” fashion, whereas nerve allografts are placed similarly to autologous grafts[24].
Clinical outcomes on collagen conduits and processed nerve allografts have been widely released over the years; even if these studies often use different scoring systems in their assessment that may render direct confrontation problematic with variable recovery rates, it has been shown that globally all studies report relevant recovery. For collagen conduits, excellent to good recovery rates ranges from 50% to 89%, while for processed decellularized human nerve allografts, it spans from 83% to 100%[36-38]. Although good outcomes have been associated with these techniques, their application is currently limited to non-critical, small caliber, nerve defects repair (< 3 cm gaps)[26,32,36,37].
A valuable solution for non-critical nerve gaps is the “muscle-in-vein” technique, which consists of an autogenous vein graft used as a conduit filled with a stripe of autologous skeletal muscle tissue[39]. The benefits of this technique lie in the composition of both vein and muscle tissue: vein walls act perfectly as a barrier against scar formation, and their non-immunogenic structure causes a less inflammatory response and possesses enough permeability to allow diffusion of neurotrophic elements[40]. Skeletal muscle tissue possesses longitudinally oriented basal lamina and extracellular matrix elements that orient and stimulate regrowing nerve fibers and, furthermore, limits the risk of vein collapse[40,41]. The surgical technique is quite easy: the vein graft ends are sutured in a similar manner as previously explained with other types of conduits[42]. Advantages of this method are many: easy availability of graft materials, no comorbidity to the donor site, immunological compatibility of the grafts, and low costs; unfortunately, the technique’s major downside is its current limitation to short nerve defects treatment (< 3 cm)[41,42].
Nerve transfers
Other options have been developed to bypass nerve gaps; nerve transfers have been traditionally employed in brachial plexus lesions and in proximal nerve injuries, but subsequently, they also gained popularity for the restoration of hand function in distal nerve injuries[43]. They are defined as the surgical coaptation of a healthy donor nerve to a recipient nerve proximal to its target, sharing a similar principle to tendon transfers in which a more useful distal function is restored by sacrificing another function that is less important to the patient[44].
This procedure has many advantages: bringing the donor nerve closer to the target reduces nerve regeneration time and accelerates functional recovery, and a motor-to-motor or a sensory-to-sensory nerve transfer can optimize regeneration potential; unlike tendon transfers, the sacrifice of a donor nerve branch with a singular function can compensate the loss of a nerve which possesses many. In addition to that, nerve transfers do not need to modify the muscle’s insertion and vector and the recovered function is closer to the original muscle function, thereby achieving synchronous physiologic motion[44-46]. Nerve transfers are indicated when nerve reconstruction would require an excessively long nerve graft, or in case of low-quality of the proximal stump, or when delayed surgery is performed; contraindications include situations in which better options are available (such as a simple end-to-end neurorrhaphy), when the excessive time between injury and reinnervation occurs, or when donor nerve motor function is below Medical Research Council grade 4[24,44,45].
It is mandatory, prior to performing the transfer, to test donor nerve function with a nerve stimulator and, at the same time, to confirm the lack of function in the recipient nerve; following the law of “donor-distal, recipient proximal”, the donor nerve is transected as distally as possible while the recipient nerve as proximally as possible in order to maximize the length of each nerve branch and to perform a tension-free repair[24,45].
In case of MN defects, restoration of the motor branch to thenar muscles is essential to the thumb’s opposition movements, and this problem can be solved by transferring the anterior interosseous nerve branch to the pronator quadratus to motor branch, usually using an interposition nerve graft; although a good match in size between the donor and recipient is achieved and effective results have been reported by different authors, the employ of nerve graft causes the loss of some regenerating axons, thus, reducing the potential of reinnervation[45,47,48].
Possible alternatives reported in the literature involve the transfer of the third lumbrical motor branch (UN) or the motor branch to the extensor digiti minimi and extensor carpi ulnaris (RN) with mixed results[43,49]; in recent years, transfer of the motor branch of the abductor digiti minimi muscle (UN) proposed by Bertelli et al. has shown encouraging results[50], while in a novel cadaveric study, Abou-Al-Shaar et al. described an interesting technique involving the motor branch to flexor digiti minimi brevi (UN), favoring this nerve due to short distance for regeneration, the synergistic activation of fifth finger flexion to enhance relearning of thumb opposition and because represents an expendable donor in the hand[46]. Transfers can be employed to restore sensibility and to decrease neuropathic pain in MN defects, in particular, to the ulnar side of the thumb and the radial side of the index finger to ensure pinch and grip functions; multiple donors have been considered depending on their availability: the best donors are the digital nerves to the fourth web space (UN), where, in order to provide limited non-critical protective sensation to the donor territories, an end-to-side coaptation can be performed; another option is the dorsal sensory branch from the RN to the thumb[45,48].
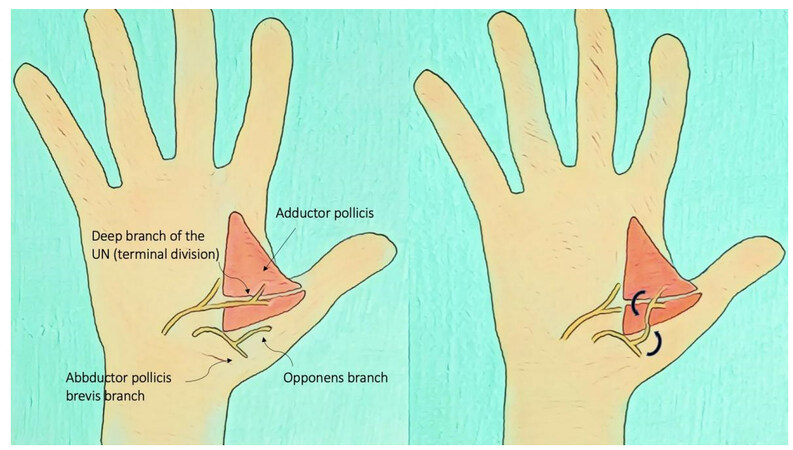
In the case of UN motor branches lesions at the hand level, Bertelli proposed transferring the motor branch of the opponens pollicis to the terminal division of the deep branch of the UN for pinch reconstruction[51] [Figure 2]; Gesslbauer, instead, proposed a nerve graft, bridging the thenar branch of the MN to the UN to enhance nerve recovery with both end-to-side coaptations[52]: in this case, the scope of the distal nerve graft is to maintain some ulnar intrinsic muscle function while awaiting recovery in the main branch of the UN. The main target and donor nerves are summarized in [Table 1].
Figure 2. Nerve transfer of the motor branch of the opponens pollicis to the terminal division of the deep branch of the UN for pinch reconstruction.
Main target and donor nerves for the management of nerve defects of the hand with nerve transfers techniques
| TARGET | DONOR | |
| MEDIAN NERVE | Motor branch to thenar muscle Sensory branch for I and II finger | Motor branch of: pronator quadratus (AIN) or third lumbrical or abductor digiti minimi or flexor digiti minimi brevi (UN) or extensor digiti minimi and extensor carpi ulnaris (RN) Digital nerves to the fourth web space (UN) or dorsal sensory branch of the thumb (RN) |
| ULNAR NERVE |
Terminal division of deep branch of the UN Deep branch of the UN |
Motor branch of the opponens pollicis Thenar branch of the MN (through nerve graft) |
Tendon transfers
When nerve structure repair is not possible or time for nerve recovery has long passed without a satisfactory result, the movement has traditionally been restored by tendon transfers; the aim of this surgery is to shift a safe, or recovered, muscle tendon to an injured site[53]. To perform a tendon transfer, the following common principles must be respected: supple joints; a healthy tissue environment; sufficient excursion and strength (at least M4) of the donor unit; the primary function of the donor unit, which should focus on a sole motor function, can be ensured by other units; the direction of the transfer should be as close to a straight line between origin and insertion as possible; lastly, the patient’s capacity and motivation to follow an extensive course of rehabilitation must be evaluated[53-55]. In MN injuries, the opposition can be restored using tendons of the flexor digitorum superficialis (FDS) of the ring or middle finger or tendon of palmaris longus (PL)[54,55]; when the FDS of the ring finger is transferred, the tendon is harvested distally in the palm and then withdrawn at the wrist. A strip of FCU attached to the pisiform can be employed to create a pulley through which the FDS tendon passes to create a line of pull in the direction of the pisiform that reproduces true opposition. Performing the Camitz opponensplasty, the PL, when present, is harvested in continuity with a strip of superficial palmar aponeurosis and attached to the insertion of the abductor on the base of the thumb’s proximal phalanx; however, since the line of pull is in the direction of the forearm, this transfer, while simple and fast, acts more like an abductorplasty instead of a true opponensplasty[54-56].
Another technique frequently used for MN palsy is the extensor indicis proprius to opponensplasty transfer, which is a popular procedure in case of high MN palsy and when the ring and middle finger FDS tendons are unavailable; it has also become preferable with respect to the superficialis tendon transfer in low MN palsies because it does not weaken grip and causes little disability[57].
Two main motor dysfunctions are produced as a result of UN injury in the hand: ring and little fingers clawing and thumb adduction impairment.
Bouvier’s test is essential to choose the best surgical option for claw correction: while the MP joint of the examined digit is retained in flexion, the patient is asked to extend the IP joint of the same digit; when it is possible to do so, the test is considered positive. In these cases, “static” procedures might be performed: Zancolli described a technique in which the MCP joint is prevented from hyperextending by advancing proximally a distally based flap of the volar plate of the MP joint and reattaching it to the metacarpal neck with sutures or anchors; Bunnell suggested an A1 and A2 pulleys release, this causes bowstringing of the flexor tendons and favors MP joint flexion; finally, Zancolli proposed a “lasso” procedure by which the FDS tendons of the ring and small fingers are divided at their insertion, passed distal to the A1 pulley and brought back, then, sewn on themselves while maintaining the MP joints in approximately 60 degrees of flexion.
When the patient is unable to extend the IP joint, the test is judged negative, and tendon transfers procedures are employed to allow a dynamic correction; one of these techniques is the modified Stiles–Bunnell tendon transfer: the FDS to the third finger is split in four, each of these slips is passed along the path of the lumbrical and inserted along the lateral bands or into the proximal phalanges[55].
Key pinch grip can be restored by acting on two fundamental elements: thumb adduction, granted by the adductor pollicis, and index finger abduction, ensured by the first dorsal interosseous. Many techniques have been described to restore thumb adduction, either by sacrificing the extensor radialis carpi brevis tendon or the ring finger’s FDS tendon or the extensor indicis tendon[58-60].
Index abduction movement might be achieved by an accessory slip of abductor pollicis longus transfer, by an extensor indicis tendon transfer, or by a palmaris longus to first dorsal interosseous tendon transfer[54,60-62].
Both extensor digiti minimi and abductor digiti minimi are considered little finger abductors; usually, the third palmar interosseous oppose their action, but in UN palsy, this muscle is paralyzed, along with the abductor digiti minimi, and the extensor digiti minimi is no longer unopposed; when a wider abduction of the fifth digit occurs, it takes the name of Wartenberg’s sign.
This deformity may be corrected either by a split of the extensor digit minimi transfer or by a junctura tendinum and medial extensor digitorum communis slip of the ring finger transfer[63].
TIMING OF NERVE SURGERY
As demonstrated, a wide range of possible surgical solutions are available for treating hand nerve defects; a multifactorial approach, considering variables such as gap length, mechanism of injury, and time of presentation, is pivotal when choosing the best option. In closed injury, whenever clinical signs consistent with a nerve lesion are detected, active surveillance is usually recommended since the first three degrees of nerve injuries have a chance to heal well on their own; however, when no signs of recovery are reported by clinical and electrophysiological findings three months after the trauma, the injured nerve should be explored to avoid progressive deterioration of both proximal and distal nerve segments and of their target organ[24,64].
On the contrary, open wounds with symptoms of nerve lesions require surgical exploration due to the high chance of complete injury; in case of a sharp cut with none or minimal crush component, good blood supply, and clean wound, primary nerve repair is the best option for restoring the function[65]. However, when immediate repair criteria are not met, a delayed repair is required, such as in cases of blunt trauma or gunshot wounds; in such situations, a waiting of up to 2-3 weeks is warranted[64].
Excessive nerve defect length prohibits an adequate tension-free neurorrhaphy; in these situations, autologous nerve grafts are the gold-standard option in peripheral nerve repairs[66]. As reported in the literature, autologous nerve grafting has better recovery results in long nerve defects (> 3 cm) [26,27]. Despite these results, however, autologous nerve grafts carry some disadvantages as stated before; considering small gaps up to 3 cm, the use of nerve guides has the same success rate as nerve autograft repair, achieving recovery in up to 69% of cases[64], and when available to the surgeon, they are a valuable resource[32,36,37].
Although many advantages have been reported using a muscle-in-vein technique, no concise conclusion is possible regarding the feasibility of this procedure for the reconstruction of long nerve defects in human patients[67]; however, its cost-effectiveness, in particular when compared to manufactured conduits, and the preservation of healthy donor nerves, makes this technique a valuable option in bridging nerve gaps up to 3 cm, with comparable outcomes to other available techniques in terms of sensibility recovery and neuropathic pain resolution[42,68].
In large nerve defects with greater regeneration times, denervated distal targets may not be successfully regenerated; unfortunately, there is tremendous variability in expert recommendations for the timing of repair[64,69]. In a 2021 systematic review focused on clinical evidence-based data on nerve repair and reconstruction, MacKay et al. provided useful considerations about this subject[64]: pure sensory nerve injuries should be treated acutely (within 14 days of injury) when possible to prevent painful neuroma formation; in injuries older than 6 months, reconstruction techniques can still be undertaken.
For mixed/motor nerve injuries, instead, the authors recommend immediate repair (within 24 hours of injury) when possible, because motor end-plate degradation limits the amount of time available for any functional motor return. For the same reason, in case of delayed presentation (more than 6 months after complete transection), efforts should be taken to provide axons to the muscle end-plates no later than 1 year and a multifactorial approach, including nerve grafting, nerve transfer, and/or tendon transfer is suggested to restore function[70].
Beyond this time period, it seems unrealistic to expect nerve surgery to work; when the degeneration of the motor end-plates, muscle atrophy, and replacement of muscle fibers by fatty tissue occurs, tendon transfer surgery becomes the only possible surgical treatment [53]. Due to different structure characteristics, it has been shown that functional sensory recovery may be achievable several years after complete transection, but the results are less predictable[64,71].
CONCLUSION
Functional recovery in hand nerve defect treatment is gradually improving in recent years. A deeper knowledge of innervation anatomical patterns combined with a classification language widely shared by specialists has provided great advantages in recognizing and guiding surgical treatment of patients whose quality of life would otherwise be compromised. Regarding surgical options, microsurgical direct nerve repair is still the gold standard for peripheral nerve repair whenever a tension-free and early repair are possible. It should be remembered, however, that nerve repair requires healthy supportive tissue to achieve optimal results. If there is a large nerve defect, an autologous nerve graft is accepted as the gold standard procedure.
Treatment of small nerve gaps is still an area of intense scientific research, but at the present time, no method has been proven superior to others; several elements, such as each technique’s feature, surgical experience, and clinical setting, must be evaluated. Numerous alternatives in nerve injury management have been provided by the advantages granted by nerve transfer procedures, and in some cases, a combination of nerve repair and immediate tendon transfer may lead to an early function recovery. Finally, when nerve surgery cannot be considered a solution, secondary surgery, such as tendon transfer procedures, still plays a significant role in improving the patient's hand function.
DECLARATIONS
Authors’ contributionsConcept and design: Titolo P, Fanecco A
Data acquisition, data analysis, manuscript preparation: Fanecco A
Critical revision and completion of manuscript: Titolo P
Availability of data and materialsNot applicable.
Financial support and sponsorshipNone.
Conflicts of interestAll authors declare that there are no conflicts of interest.
Ethical approval and consent to participateNot applicable.
Consent for publicationNot applicable.
Copyright© The Author(s) 2023.
REFERENCES
1. Jain A, Dunlop R, Hems T, Tang JB. Outcomes of surgical repair of a single digital nerve in adults. J Hand Surg Eur Vol 2019;44:560-5.
2. Taylor CA, Braza D, Rice JB, Dillingham T. The incidence of peripheral nerve injury in extremity trauma. Am J Phys Med Rehabil 2008;87:381-5.
3. Padovano WM, Dengler J, Patterson MM, et al. Incidence of nerve injury after extremity trauma in the United States. Hand 2022;17:615-23.
4. Carter GT, Robinson LR, Chang VH, Kraft GH. Electrodiagnostic evaluation of traumatic nerve injuries. Hand Clin 2000;16:1-12, vii.
5. McGillivray MK, Haldane C, Doherty C, Berger MJ. Evaluation of muscle strength following peripheral nerve surgery: a scoping review. PM R 2022;14:383-94.
6. DaSilva MF, Moore DC, Weiss AP, Akelman E, Sikirica M. Anatomy of the palmar cutaneous branch of the median nerve: clinical significance. J Hand Surg Am 1996;21:639-43.
7. Richards O, Border S, Bolton C, Webb AL. The palmar cutaneous branch of the median nerve: a detailed morphometric study. Forensic Med Anat Res 2014;2:101-106.
8. Dowdy PA, Richards RS, McFarlane RM. The palmar cutaneous branch of the median nerve and the palmaris longus tendon: a cadaveric study. J Hand Surg Am 1994;19:199-202.
9. Hobbs RA, Magnussen PA, Tonkin MA. Palmar cutaneous branch of the median nerve. J Hand Surg Am 1990;15:38-43.
10. al-Qattan MM. Anatomical classification of sites of compression of the palmar cutaneous branch of the median nerve. J Hand Surg Br 1997;22:48-9.
11. Smith JL, Ebraheim NA. Anatomy of the palmar cutaneous branch of the median nerve: a review. J Orthop 2019;16:576-9.
12. Wynter S, Dissabandara L. A comprehensive review of motor innervation of the hand: variations and clinical significance. Surg Radiol Anat 2018;40:259-69.
13. Corroller T, Bauones S, Acid S, Champsaur P. Anatomical study of the dorsal cutaneous branch of the ulnar nerve using ultrasound. Eur Radiol 2013;23:2246-51.
14. Bianchi S, Beaulieu JY, Poletti PA. Ultrasound of the ulnar-palmar region of the wrist: normal anatomy and anatomic variations. J Ultrasound 2020;23:365-78.
15. Costa AL, Natsis K, Romeo M, et al. Topography of the deep branch of the ulnar nerve between genders: a cadaveric study with potential clinical implications. J Plast Surg Hand Surg 2023;57:178-80.
16. Colonna MR, Piagkou M, Monticelli A, et al. Lumbrical muscles neural branching patterns: a cadaveric study with potential clinical implications. Hand 2022;17:839-47.
17. Smith JL, Siddiqui SA, Ebraheim NA. Comprehensive summary of anastomoses between the median and ulnar nerves in the forearm and hand. J Hand Microsurg 2019;11:1-5.
18. Martin SP, Schauer KT, Czyrny JJ, Ablove RH. Electrophysiological findings in common median-ulnar nerve interconnections and their clinical implications. J Hand Surg Am 2019;44:884-94.
19. Wali A, Ahmed R, Khan S. Electrophysiological evidence of the Riche-Cannieu anastomosis in the hand and its diagnostic implications; 2 case reports. Clin Neurophysiol Pract 2017;2:8-11.
21. Seddon HJ, Medawar PB, Smith H. Rate of regeneration of peripheral nerves in man. J Physiol 1943;102:191-215.
22. SUNDERLAND S. A classification of peripheral nerve injuries producing loss of function. Brain 1951;74:491-516.
23. Wang ML, Rivlin M, Graham JG, Beredjiklian PK. Peripheral nerve injury, scarring, and recovery. Connect Tissue Res 2019;60:3-9.
24. Dahlin LB, Wiberg M. Nerve injuries of the upper extremity and hand. EFORT Open Rev 2017;2:158-70.
25. Fox IK, Mackinnon SE. Adult peripheral nerve disorders: nerve entrapment, repair, transfer, and brachial plexus disorders. Plast Reconstr Surg 2011;127:105e-18e.
26. Grinsell D, Keating CP. Peripheral nerve reconstruction after injury: a review of clinical and experimental therapies. Biomed Res Int 2014;2014:698256.
27. Tezcan AH. Peripheral Nerve Injury and Current Treatment Strategies. In: Mauricio AC, editor. Peripheral nerve regeneration - from surgery to new therapeutic approaches including biomaterials and cell-based therapies development. InTech; 2017.
28. Smetana BS, Cao J, Merrell GA, Greenberg JA. Testing of direct neurorrhaphy strain. J Hand Surg Am 2019;44:615.e1-6.
29. Koopman JE, Duraku LS, de Jong T, et al. A systematic review and meta-analysis on the use of fibrin glue in peripheral nerve repair: can we just glue it? J Plast Reconstr Aesthet Surg 2022;75:1018-33.
30. Sallam A, Eldeeb M, Kamel N. Autologous fibrin glue versus microsuture in the surgical reconstruction of peripheral nerves: a randomized clinical trial. J Hand Surg Am 2022;47:89.e1-89.e11.
31. Manoli T, Schiefer JL, Schulz L, Fuchsberger T, Schaller HE. Influence of immobilization and sensory re-education on the sensory recovery after reconstruction of digital nerves with direct suture or muscle-in-vein conduits. Neural Regen Res 2016;11:338-44.
34. Agarwal R, Agarwal D, Agarwal M. Approach to mutilating hand injuries. J Clin Orthop Trauma 2021;15:172-5.
35. Battiston B, Titolo P, Ciclamini D, Panero B. Peripheral nerve defects: overviews of practice in Europe. Hand Clin 2017;33:545-50.
36. Rebowe R, Rogers A, Yang X, Kundu SC, Smith TL, Li Z. Nerve repair with nerve conduits: problems, solutions, and future directions. J Hand Microsurg 2018;10:61-5.
37. Rbia N, Bulstra LF, Saffari TM, Hovius SER, Shin AY. Collagen nerve conduits and processed nerve allografts for the reconstruction of digital nerve gaps: a single-institution case series and review of the literature. World Neurosurg 2019;127:e1176-84.
38. Rinker BD, Ingari JV, Greenberg JA, et al. Outcomes of short-gap sensory nerve injuries reconstructed with processed nerve allografts from a multicenter registry study. J Reconstr Microsurg 2015;31:384-90.
39. Brunelli GA, Battiston B, Vigasio A, Brunelli G, Marocolo D. Bridging nerve defects with combined skeletal muscle and vein conduits. Microsurgery 1993;14:247-51.
40. Sabongi RG, Fernandes M, Dos Santos JB. Peripheral nerve regeneration with conduits: use of vein tubes. Neural Regen Res 2015;10:529-33.
41. Meek MF, Varejão AS, Geuna S. Use of skeletal muscle tissue in peripheral nerve repair: review of the literature. Tissue Eng 2004;10:1027-36.
44. Lee SK, Wolfe SW. Nerve transfers for the upper extremity: new horizons in nerve reconstruction. J Am Acad Orthop Surg 2012;20:506-17.
45. Moore AM, Franco M, Tung TH. Motor and sensory nerve transfers in the forearm and hand. Plast Reconstr Surg 2014;134:721-30.
46. Abou-Al-Shaar H, Dorius GT, Morton DA, Mahan MA. Distal nerve transfer for thenar palsy: a cadaveric study. Clin Anat 2020;33:414-8.
47. Frank K, Englbrecht M, Koban KC, et al. Nerve transfer of the anterior interosseous nerve to the thenar branch of the median nerve - an anatomical and histological analysis. J Plast Reconstr Aesthet Surg 2019;72:751-8.
48. Sassu P, Libberecht K, Nilsson A. Nerve transfers of the forearm and hand: a review of current indications. Plast Aesthet Res 2015;2:195-201.
49. Schultz RJ, Aiache A. An operation to restore opposition of the thumb by nerve transfer. Arch Surg 1972;105:777-9.
50. Bertelli JA, Soldado F, Rodrígues-Baeza A, Ghizoni MF. Transfer of the motor branch of the abductor digiti quinti for thenar muscle reinnervation in high median nerve injuries. J Hand Surg Am 2018;43:8-15.
51. Bertelli JA, Soldado F, Rodrígues-Baeza A, Ghizoni MF. Transferring the motor branch of the opponens pollicis to the terminal division of the deep branch of the ulnar nerve for pinch reconstruction. J Hand Surg Am 2019;44:9-17.
52. Gesslbauer B, Furtmüller GJ, Schuhfried O, et al. Nerve grafts bridging the thenar branch of the median nerve to the ulnar nerve to enhance nerve recovery: a report of three cases. J Hand Surg Eur Vol 2017;42:281-5.
53. Pierrart J, Aumar A, Masmejean E. Palliative surgery: when? which technique? basic principlesChirurgie palliative: quand? quelle technique? principes de base. Hand Surg Rehabil 2022;41S:S5-S10.
54. Hentz VR. Tendon transfers after peripheral nerve injuries: my preferred techniques. J Hand Surg Eur Vol 2019;44:775-84.
55. Gardenier J, Garg R, Mudgal C. Upper extremity tendon transfers: a brief review of history, common applications, and technical tips. Indian J Plast Surg 2020;53:177-90.
56. Gaisne E, Bellemère P, Kerjean Y, Loubersac T, Chaves C. Restoration of thumb opposition (opponensplasty). Hand Surg Rehabil 2022;41S:S105-11.
57. Al-Qattan MM. Extensor indicis proprius opponensplasty for isolated traumatic low median nerve palsy: a case series. Can J Plast Surg 2012;20:255-7.
58. Smith RJ. Extensor carpi radialis brevis tendon transfer for thumb adduction--a study of power pinch. J Hand Surg Am 1983;8:4-15.
59. Edgerton MT, Brand PW. Restoration of abduction and adduction to the unstable thumb in median and ulnar paralysis. Plast Reconstr Surg 1965;36:150-64.
61. Neviaser RJ, Wilson JN, Gardner MM. Abductor pollicis longus transfer for replacement of first dorsal interosseous. J Hand Surg Am 1980;5:53-7.
62. Hirayama T, Atsuta Y, Takemitsu Y. Palmaris longus transfer for replacement of the first dorsal interosseous. J Hand Surg Br 1986;11:84-6.
63. Voche P, Merle M. Wartenberg's sign. a new method of surgical correction. J Hand Surg Br 1995;20:49-52.
64. MacKay BJ, Cox CT, Valerio IL, et al. Evidence-based approach to timing of nerve surgery: a review. Ann Plast Surg 2021;87:e1-e21.
65. Siemionow M, Brzezicki G. Chapter 8 current techniques and concepts in peripheral nerve repair. Elsevier; 2009. pp. 141-72.
66. Namazi H, Sobhani A, Gholamzadeh S, Dehghanian A, Dehghani Nazhvani F. Donor nerve graft assessment for covering thumb nerve defects: a cadaveric study. J Orthop Surg Res 2020;15:456.
67. Heinzel JC, Quyen Nguyen M, Kefalianakis L, et al. A systematic review and meta-analysis of studies comparing muscle-in-vein conduits with autologous nerve grafts for nerve reconstruction. Sci Rep 2021;11:11691.
68. Mohammadi J, Delaviz H, Mohammadi B, Delaviz H, Rad P. Comparison of repair of peripheral nerve transection in predegenerated muscle with and without a vein graft. BMC Neurol 2016;16:237.
69. Wang E, Inaba K, Byerly S, et al. Optimal timing for repair of peripheral nerve injuries. J Trauma Acute Care Surg 2017;83:875-81.
70. Reyad KA, Behiri AM, El Lamie KK, Sayed MA, Abd Elsabour Sabah HM. Immediate tendon transfer with nerve repair in low combined ulnar and median nerve injury. Plast Reconstr Surg Glob Open 2021;9:e3597.
Cite This Article
Export citation file: BibTeX | RIS
OAE Style
Titolo P, Fanecco A. The treatment of nerve defects. Plast Aesthet Res 2023;10:21. http://dx.doi.org/10.20517/2347-9264.2022.113
AMA Style
Titolo P, Fanecco A. The treatment of nerve defects. Plastic and Aesthetic Research. 2023; 10: 21. http://dx.doi.org/10.20517/2347-9264.2022.113
Chicago/Turabian Style
Titolo, Paolo, Andrea Fanecco. 2023. "The treatment of nerve defects" Plastic and Aesthetic Research. 10: 21. http://dx.doi.org/10.20517/2347-9264.2022.113
ACS Style
Titolo, P.; Fanecco A. The treatment of nerve defects. Plast. Aesthet. Res. 2023, 10, 21. http://dx.doi.org/10.20517/2347-9264.2022.113
About This Article
Special Issue
Copyright
Data & Comments
Data

 Cite This Article 8 clicks
Cite This Article 8 clicks










Comments
Comments must be written in English. Spam, offensive content, impersonation, and private information will not be permitted. If any comment is reported and identified as inappropriate content by OAE staff, the comment will be removed without notice. If you have any queries or need any help, please contact us at support@oaepublish.com.