Ischemic complications of dermal fillers
Abstract
Dermal fillers have become increasingly popular as a cosmetic treatment for facial rejuvenation. Although these injections are generally considered to be safe, as the number of injections has increased, so has the rate of complications. Ischemic complications of fillers include vision loss, ophthalmoplegia, skin necrosis, and cerebral infarction. Knowing the anatomy well is critical to optimally prevent and manage these serious complications. Prevention includes knowledge of the vascular anatomy of the facial area, as well as certain injection techniques such as aspiration, use of a smaller needle, and adoption of a larger cannula. The use of ultrasound has been a recent innovation in preventing and treating filler complications as well. The reversibility of fillers should also be considered when choosing a filler. Some hyaluronic acid (HA) fillers, including the newer ones on the market, are difficult to reverse and non-HA fillers and fat are irreversible. This review aims to discuss facial anatomy, the various ischemic filler complications, the prevention and management of these complications, and the relatively recent use of imaging as an adjunct.
Keywords
INTRODUCTION
Dermal fillers are used to treat changes commonly seen with aging in the face. Since bovine collagen became FDA approved for cosmetic use in 1981, soft tissue fillers have consistently increased in popularity, with the number of procedures increasing by 50% from 2015 to 2019[1,2]. With this increase in filler injections, there has been a significant increase in the number of products available on the market, with each filler having its own intrinsic properties[3]. While filler injections are generally considered to be a safe procedure, there are still many complications that have been documented and studied in the literature.
The most devastating filler complications are ischemic, which include irreversible vision loss, ophthalmoplegia, and skin necrosis, among other serious complications[4-6]. The incidence of vascular occlusion appears to be up to 3 in 1000 injections[7], and a total of at least 190 cases of blindness have been reported in literature as reviewed by Chatrath et al. as of 2019[8]. As of January 2022, we have found at least 211 cases, with Table 1 noting some of the more recent cases. Some cases, like a report of bilateral blindness after nasal augmentation with calcium hydroxylapatite[9], were found to have been missed in previous reviews. Many more cases of blindness have likely gone unreported, so the true incidence is unknown. While the reported cases appear to be a small percentage of the total injections, the rate of this complication appears to be increasing[10,11]. Although the incidence of these complications is low, their severity warrants further studies into how to improve management and prevent these complications from occurring.
Recent case reports of vision loss following filler injections
| Author | Demographics | Material | Site of injection | Eye | Symptoms | Time to treatment onset | Treatment | Outcome |
| Davidova et al. (2022)[117] | 43-year-old female | Hyaluronic acid | Glabella | Left | Left sided vision loss (NLP) Ptosis Ophthalmoplegia Swelling on left forehead and upper lid Left RAPD | 1 h | Ocular massage Aspirin Tinzaparin sodium Methylprednisolone Antiseptic compresses Three hyaluronidase injections in the injection area | Vision remained at NLP at 6 weeks Redness and surface irregularity on the forehead and madarosis on the inner third of the upper lid Restricted EOM at 6 week follow up |
| Wu et al. (2021)[55] | 49-year-old female | Poly-L-Lactic acid | Temporal region | Right | Right sided central visual defect Ocular pain Dizziness Nausea Photopsia Right RAPD | 1 h | Ocular massage Topical brimonidine Hyperbaric oxygen therapy twice daily for 5 days Dual-antiplatelet treatment (from prior) | Vision remained at NLP in the right eye at 1 year |
| Danks et al. (2021)[118] | 38-year-old female | Hyaluronic acid | Right side of nose | Right | Right sided visual loss (NLP) Right sided headache Skin pallor Right RAPD | 1) Immediately post injection 2) 4 h after injection | 1) 675 IU hyaluronidase to the filler site 2) 3 injections of 1500 IU hyaluronidase; one peribulbar and 2 extraorbital Unknown dose aspirin | Vision improved from NLP to CF at 4 h and 20/20 at 1 month follow up Resolution of right RAPD |
| Nguyen et al. (2021)[119] | 27-year-old female | Hyaluronic acid | Nasal dorsum | Right | 1) Right sided vision loss (NLP) Right sided ptosis Right sided ophthalmoplegia Frontal and nasal ecchymosis Headache Pain 2) 13 h later, patient developed a headache with ocular pain Right sided vision loss worsened from CF at 1 meter to NLP | 4 h | 1) 1500 IU hyaluronidase to frontal and nasal areas; 750 IU hyaluronidase retrobulbar; 1500 IU intra-arterial hyaluronidase into the right ophthalmic artery 2) Alteplase 8 mg 1500 IU hyaluronidase Heparin 5000 IU/day Aspirin Nitroglycerin patches Corticosteroid Antibiotic therapy | 1) Vision improved from NLP to CF at 1 meter 2) Visual acuity improved from NLP to 20/50 at 3 months Skin ecchymosis fully recovered at 3 months Ophthalmoplegia and eyelid ptosis recovered at 3 months |
| Lee et al. (2021)[102] | 39-year-old female | Hyaluronic acid | Glabella | Left | Left sided vision loss (NLP) Left sided ocular pain Drowsy mental status Motor weakness in right upper and lower limbs Dysarthria, hypesthesia, right arm dominant quadriparesis Left sided ophthalmoplegia and ptosis | Unknown | Methylprednisolone 1 g for 5 days | Vision remained at NLP at 6 weeks Ophthalmoplegia and ptosis partially recovered at 6 weeks |
| Moore et al. (2021)[120] | 59-year-old female | Hyaluronic acid | Glabella; nasal dorsum | Right | Right sided vision loss (NLP) Dizziness Nausea Right frontal headache Right RAPD Subclinical punctate strokes Right forehead livedo reticularis | Unknown | Unknown volume hyaluronidase at unknown location 1100 IU retrobulbar hyaluronidase Ocular massage 250 IU of hyaluronidase in glabella and right forehead Warm compresses Topical Nitroglycerin 2% ointment 650 mg aspirin | Vision remained at NLP at 1 week follow up Right forehead had a 1 × 2 cm eschar at 1 week follow up |
| Eldweik (2021)[121] | 32-year-old female | Hyaluronic acid | Nasal bridge | Left | Left sided vision loss (NLP) Swelling and tenderness around left eye Bluish discoloration of facial skin Dull, aching pain Limited EOM Ophthalmoplegia Left blepharoptosis | 1) Immediately post injection 2) Less than 1 h | 1) 40 IU hyaluronidase at site of injection 2) 300 IU peribulbar injection of hyaluronidase Methylprednisolone 1 g for 5 days Aspirin Antibiotic coverage Antibiotic cream | Vision remained at NLP at 8 weeks Skin necrosis resolved with scarring at 8 weeks Left EOM and blepharoptosis improved at 8 weeks |
| Jolly et al. (2021)[122] | 29-year-old female | Unknown | Nasal bridge to tip | Left | Left sided vision loss (LP) only in upper temporal quadrant Left RAPD Left sided ptosis Left sided ophthalmoplegia Reduced EOM Left periorbital ecchymosis Left subconjunctival hemorrhage | 6 h | Iopidine 1% 10 min ocular massage 1500 IU hyaluronidase sub-Tenon’s injection Anterior chamber paracentesis 250 mg oral acetazolamide | Vision worsened from LP to NLP at 3 months Improvement in EOM and ptosis at 3 months |
| Hung et al. (2021)[54] | 31-year-old female | Hyaluronic acid | Nasal dorsum | Right | Right sided vision loss (NLP) Severe sharp pain in retro-orbital area | 1) Immediately after injection 2) Unknown | 1) Unknown dose of hyaluronidase in treated area 2) 300 mL mannitol intravenously High-flow O2 3 cycles of ocular massage for 10 s every 4 h 0.15% topical brimonidine 3x daily 250 mg oral acetazolamide 4x daily Aspirin daily 250 mg intravenous methylprednisolone q6h HBOT 2.5 ATA for 90 min daily for 3 weeks | Vision remained at NLP at 5 months Superficial skin necrosis development at 2-week over nasion and rhinion which later resolved at 4-month follow up |
| Zhang et al. (2021)[123] | 61-year-old female | Hyaluronic acid | Radix nasi | Right | Right sided severe blurred vision Right sided ptosis Right sided ophthalmoplegia Swelling in right eye Pain in right eye Nausea Vomiting Chest tightness | 22 h | Glucocorticoids Mannitol Right anterior chamber puncture Nutritional nerve therapy Endovascular hyaluronidase application through angiography* | Vision remained at NLP at 8 months |
| Zhang et al. (2021)[123] | 31-year-old female | Hyaluronic acid | Glabella and Forehead | Right | Right sided vision loss Ophthalmoplegia Headache Eye pain Chest tightness Nausea Vomiting Sluggishness | 30 min | Unknown hyaluronidase dose at the site of injection IV infusion of mannitol Endovascular hyaluronidase application through angiography* | Vision improved from NLP to slight light sensation at 8 months |
| Zhang et al. (2021)[123] | 31-year-old female | Hyaluronic acid | Apex nasi | Left | Left sided vision impairment Ptosis Local nose skin pallor Nausea Vomiting Incontinence | 4.5 h | Subcutaneous injection of hyaluronidase IV infusion of mannitol Endovascular hyaluronidase application through angiography* | Vision remained at NLP at 3 months |
| Zhang et al. (2021)[123] | 46-year-old female | Hyaluronic acid | Palpebra superior | Right | Right sided immediate vision loss Sharp pain at injection site Ptosis | 4 h | Subcutaneous hyaluronidase Sublingual administration of nitroglycerin Endovascular hyaluronidase application through angiography* | Vision remained at NLP at 3 months |
| Liu et al. (2020)[124] | 29-year-old female | Autologous fat | Forehead | Left | Left sided vision loss (NLP) Ocular pain Left RAPD Decreased IOP Nausea Vomiting Numbness and weakness of right limbs (Grade 2-3) with parietal lobe hyperintense lesion | Immediately after injection | IV infusion of dextran glucose with dexamethasone and mannitol | Vision remained at NLP at 3 months |
| Liu et al. (2020)[124] | 46-year-old female | Autologous fat | Forehead | Left | Left sided vision loss (NLP) Ocular pain Nausea Vomiting Decreased IOP Congestion and swelling of injection site Left exotropia (10 degrees) Limited EOM | Immediately after injection | IV infusion of dexamethasone and energy mixture | Vision remained at NLP at 3 months |
| Liu et al. (2020)[124] | 38-year-old female | Autologous fat | Forehead | Left | Left sided vision loss (NLP) Ocular pain Nausea Vomiting Left RAPD Decreased IOP | Immediately after injection | IV infusion of dexamethasone and cold compression | Vision remained at NLP at 3 months Chromatosis at 3 months in the left forehead skin |
| Conovaloff et al. (2020)[125] | 59-year-old female | Hyaluronic acid | Supraorbital region | Right | Right sided vision loss (NLP) Dizziness Nausea Frontal headache Right RAPD Punctate infarcts in right frontal and occipital lobes | 1) Immediately after injection 2) 120 min | 1) Hyaluronidase injection to affected area 2) Nitroglycerin paste Warm compress Eye massage Aspirin 325 mg | Vision remained at NLP at 2 months |
| Karam et al. (2020)[43] | 61-year-old female | Platelet-rich plasma | Glabella | Left | 1) Left sided vision loss (NLP) Dizziness Vomiting Glabellar bruising Hypoaesthesia in distribution of first trigeminal branch on left side 2) 1 month later, ulceration and skin necrosis in injection area | Unknown | Unknown | Pale left optic disc with pigment, pigmentation of peripheral retina and macular fibrosis at 1 month Retinal detachment in left eye at 8 months Persistent scarring in the injection area at 8 months |
| Karam et al. (2020)[43] | 63-year-old female | Platelet-rich plasma | Forehead | Right | Right sided vision loss Dizziness Tinnitus Vomiting Iris depigmentation | Unknown | Unknown | Vision was NLP at 3-week visit |
| Karam et al. (2020)[43] | 52-year-old female | Platelet-rich plasma | Nasolabial fold Glabella | Right | Right sided vision loss Pain Incomplete oculomotor nerve palsy Low intraocular pressure | Unknown | Unknown | Vision was NLP at 24 h Necrosis of forehead, right periorbital region, right cheek, and right nasal area at 1 month |
| Karam et al. (2020)[43] | 50-year-old female | Platelet-rich plasma Platelet gel injection | Forehead Glabella Right external canthus | Right | Right sided vision loss Transient blue vision preceding loss of vision Headache Nausea Ptosis Urinary urgency | Unknown | Unknown | Vision was NLP at 3 weeks along with complete right oculomotor nerve palsy |
| Wibowo et al. (2019)[62] | 40-year-old female | Hyaluronic acid | Nasal dorsum | Right | Right sided blurring of vision (LP) Right sided eyelid ptosis Periorbital swelling, chemosis, conjunctival congestion Discoloration and pustules of nose tip, nose bridge, columella, glabella, forehead, and bilateral medial cheeks Right sided pain | 1) Immediately after injection 2) 3 days | 1) Local massage 30 IU of hyaluronidase at unknown location 2) 1500 IU Hyaluronidase in the ischemic zone (5 doses) Aspirin Antibiotics 900 IU Hyaluronidase retrobulbar (2 doses) | Improvement in eyelid ptosis at 3 weeks Vision fully recovered from LP at 3 months Minimal skin deformity at 3 months |
| Vu et al. (2018)[25] | 51-year-old female | Calcium Hydroxyapatite | Glabella Dorsum of the nose | Right | Right sided vision loss (NLP) Skin discoloration of right forehead, glabella, dorsum of the nose to nasal tip, medial cheek Nausea Vomiting Headache Right RAPD | 12 h | 1200 IU retrobulbar injections as 3 serial bolus injections Ocular massage initiated Oral prednisone 60 mg for 3 days 150 IU/day hyaluronidase for skin ischemia Nitroglycerin paste applied to skin Oral aspirin daily Hyperbaric oxygen therapy for skin necrosis | Vision improved from NLP to LP at 3 month follow up Glabella and forehead had erythematous, depressed scars at 3 months |
| Ramesh et al. (2018)[126] | 23-year-old male | Hyaluronic acid | Nasal dorsum | Right | 1) Right sided vision loss (NLP) Cold sensation in his face and brow Drooping of the right upper eyelid Pustular skin lesions across brow, nose, and face Eye pain 2 days after infection 2) Right sided vision loss (NLP) Retrobulbar ache Reduced EOM Edematous and ptotic eyelid Conjunctival injection and subconjunctival hemorrhage Anterior chamber hypopyon Reduced IOP (8 mm Hg) 1 mm proptosis with resistance to retropulsion Fixed dilated pupil Decreased right V1 sensation Crusted lesions on forehead and nose | 1) Unknown time 2) 7 days | 1) IV steroids Ocular massage Antibiotics 2) 1200 IU hyaluronidase injected into the orbital apex 600 IU injected into the skin lesions | Vision remained at NLP at 21 days Improvement in ptosis and EOM at 21 days |
| Chen et al. (2018)[127] | 31-year-old female | Hyaluronic acid | Right front and eyebrow | Right | Right sided vision loss (NLP) Ocular pain Ophthalmoplegia Ptosis Limited EOM Chills Fatigue Nausea Dizziness | 1) Immediate hyaluronidase injection 2) 24 h to thrombolytic therapy 3) 6 days to rest of treatment | 1) Unknown injection of hyaluronidase in forehead and eyebrow Unknown dosage of retrobulbar anisodamine Anterior chamber puncture 2) Thrombolytic therapy of urokindase 3) 750 IU hyaluronidase peribulbar/retrobulbar for 5 days | Vision remained at NLP at 3 months Complete recovery in ptosis and EOM at 3 months |
| Ansari et al. (2018)[101] | 20-year-old female | Hyaluronic acid | Glabella | Right | Right sided vision loss (NLP) Infarcts of parietal lobes Violaceous pigmentation on the tip and right ala of her nose | Unknown; patient came to clinic for 2nd opinion | Aspirin 325 mg Prednisone taper | Unknown |
| Kalyam et al. (2017)[128] | 49-year-old female | Platelet-rich Plasma (PRP) | Forehead rhytids | Right | Right sided vision loss (NLP) Pain and fullness behind right eye Right RAPD Limited EOM Exotropia and Hypotropia Ecchymosis and induration above medial brow Subacute infarctions in frontal, parietal, and occipital lobes | Approximately 24 h | Ocular massage Topical timolol 0.5% Brimonidine 0.2% Oral steroids Intravenous antibiotics | Vision remained at NLP at 1 year Improvement in EOM starting week 2 Scarring and hard nodules in the right glabellar region at 1 year |
| Kim et al. (2013)[9] | 30-year-old male | Calcium hydroxyapatite | Nose | Bilateral | Vision loss bilaterally (NLP) Blepharoptosis Total ophthalmoplegia Central skin necrosis Reticular pattern affecting nose and frontal area Conjunctival injection and emboli along conjunctival vessels | Unknown | Unknown | Unknown |
| Roberts et al. (2011)[45] | 43-year-old male | Poly-(L)-Lactic acid | Periorbital region | Left | Left sided vision loss (LP) Orbital pain Ptosis Fixed and dilated pupil Decreased IOP Enophthalmos Ophthalmoplegia Conjunctival chemosis, paracentral epithelial defect of the cornea with corneal edema, 2-3+ cellular reaction and pigment | Unknown | Unknown | By self-report, vision remained depressed By self-report, improvement in ocular movements and ptosis |
This literature review aims to discuss the various ischemic complications seen after injection and their management. Articles until January 2022 were included with a specific focus on recently published literature. This review focuses on the anatomy of the face and ischemic complications seen after filler injections, discusses the management of these complications, reviews prevention techniques, and examines the relatively recent use of imaging as an adjunct.
ANATOMY
The anatomy of the face is complex and has a considerable amount of variation. During a glabellar injection, the supratrochlear and supraorbital arteries are at the highest risk[12]. Both of these arteries supply the superomedial aspects of the forehead and provide retrograde flow to the ophthalmic artery
Figure 1. Representative figure of the arteries of the face, ocular, and nasal area. Shaded areas represent communication between the external carotid system and the end branches of the ophthalmic artery (OA) that are implicated in filler induced blindness. This includes the angular branch (AngA) of the facial artery (FA), the transverse facial artery (TFA) that arises from the superficial temporal artery (STempA) and the deep temporal branch (DTA) of the maxillary artery (MA) in the shaded zones. The frontal branches of the superficial temporal artery (FBSTA) anastomoses with the supraorbital (SOA) and supratrochlear arteries (STA), but the FBSTA has not been directly implicated in blindness after filler injections. Other vessels mentioned are the internal carotid artery (IC), external carotid artery (EC), inferior labial artery (ILA), superior labial artery (SLA), inferior alar artery (AIA), dorsal nasal artery (DNA), zygomaticofacial artery (ZF), zygomaticotemporal artery (ZT), and lacrimal artery (LA). Figure and caption reproduced with permission from Goodman et al. 2020 by permission of Oxford University Press on behalf of The Aesthetic Society[14].
The facial artery continues midface as the angular artery immediately subjacent to the nasolabial fold
In cases of temporal hollowing, the frontal branch of the superficial temporal artery and the middle temporal vein should be avoided [Figure 1][12]. The vein lies within the temporal fat pad, while the artery lies within the temporoparietal fascia and travels superficially to above the lateral eyebrow[12,18]. The superficial temporal artery also anastomoses with the supraorbital, supratrochlear and zygomaticotemporal arteries, providing a pathway into the central retinal artery[13]. The author prefers injecting using high G’ fillers deep on top of the bone in this high-risk area; however, the exit of the zygomaticotemporal artery traverses into the orbit just above the zygomatic arch, usually more anteriorly, and may also be a source of orbital ischemia. Poly-L-lactic acid is another alternative due to the volume available when diluted 8:1 or 9:1 with sterile water and lidocaine. The less viscous preparation is easier to see reflux, if in an artery.
When injecting the cheek, one should be aware of the infraorbital bundle, which lies approximately 1 centimeter below the orbital rim at the medial limbus[19], the transverse facial artery along the zygoma, and the zygomaticofacial artery higher up on the zygomatic arch laterally [Figure 1]. Two main danger zones exist in the infraorbital and cheek area[20]. Periosteal injections for tear-trough deformities or infraorbital hollow correction can be dangerous due to anastomoses of the nasal branch of the infraorbital artery with the supratrochlear artery, dorsal nasal artery, or angular artery[20]. Secondly, for cheekbone enhancement fillers, superficial injections may cause problems with the cutaneous perforations of the zygomaticomalar branch of the infraorbital artery[20]. In these areas, a high G’ product is recommended[12]. Additionally, for tear trough deformities, retromuscular, pre-orbital microfat injections may be considered, while for cheekbone enhancement, injections should be performed in supraperiosteal layers. However, fat injections are not reversible, and there is a wide variation in how HA fillers can be reversed[21-23]. For example, Restylane-Lyft for the cheek takes very little hyaluronidase to dissolve, while Voluma and RHA4 are very difficult to reverse[21-23]. Calcium hydroxylapatite is also not reversible, although some have seen improvement with simple saline diffusion[24], hyaluronidase[25], sodium thiosulfate[26], steroids[27], and 5-fluorouracil injections[28].
Given the vascularity of the nose, filler injections in the nasal area are commonly associated with complications[12,29,30]. Although the major nasal arteries at risk for complications are the lateral nasal artery and dorsal nasal artery, the presence of several anastomoses in the nose also predisposes to blood flow that can be reversed with filler injection[11]. The vasculature typically lies in the subdermal plane above the superficial musculoaponeurotic system [Figure 1][11,12]. The tip and ala of the nose are most commonly prone to necrosis secondary to compression or vascular injury[12]. If one were willing to risk injecting this area, one author recommends reassessing the patient 15 min after injection to check for vascular compromise[12].
The superior labial artery (SLA) and inferior labial artery (ILA) also have variability in their course and depth[31]. While the SLA is not very likely to be found subcutaneously at the vermillion border, the vasculature is superficial in the midline and Cupid’s area, which can carry a high risk[31]. Additionally, the lips become thinner with aging, which may predispose the arteries to intravascular injection[31]. Therefore, for lip injections, injections should be limited to approximately 3 mm depth in order to avoid the SLA and ILA that course deeper within the lip[12].
In cases of jawline contouring, one study found an anatomic variation in which the transverse facial artery travels from the masseter muscle to the angular artery and dorsal nasal artery[32]. This could be one pathway for how lateral face injections, including masseter and jawline contouring, could lead to blindness[32].
COMPLICATIONS
Vision loss
As cited in the introduction and the Table, there are at least 211 cases of blindness reported in the English literature as of January 2022, and the actual number is likely many folds higher, since most cases are not published in literature. The presentation of a patient with vascular occlusion of the ophthalmic artery involves blindness and periocular symptoms, usually very soon after filler injection, and can present with concurrent ptosis and ophthalmoplegia[29,34]. In a recent review paper, 35 out of 39 cases had immediate vision loss symptoms and 2 cases developed symptoms within 10 min[29]. However, in several cases, the symptoms developed a day after injection[29]. The etiology of this vision loss is usually due to retrograde arterial embolism into branches of the ophthalmic artery, including the retinal branches[35-37]. The glabella, temple, and nasolabial folds have vasculature that commonly anastomoses to the ophthalmic artery[38]. Thus, the most common locations that cause vision changes are the nasal region, followed by the glabella, forehead, and nasolabial folds[5,39]. The most common occluded vessels are the ophthalmic artery, central retinal artery, branch retinal artery, and naso ciliary artery[29,30]. Occlusion of the ophthalmic artery is usually secondary to injections in the nose, while occlusion of the retinal artery is secondary to glabellar injections[30]. Given the various anastomoses in the different facial arteries, there are several injection locations that can cause ocular complications.
A recent study found that filler particles disintegrate into smaller particles immediately after injection, supporting the hypothesis that emboli, rather than a column of filler, cause an obstruction[40]. Another study further supported this idea by showing that the force required to push a column of filler retrograde was higher than the normal injection force[41]. However, one study also found that only 0.085 mL was required to fill the supratrochlear artery from the glabella to the ophthalmic and central retinal artery bifurcation, highlighting that even a small volume of filler could cause this complication[42]. Per the 48 published case reports by Beleznay et al. of filler-induced vision changes, 81% of cases were treated with hyaluronic acid filler followed by calcium hydroxylapatite (10.4%), and one case each was from autologous fat and poly-L-lactic acid (PLLA)[5]. This is likely secondary to the fact that hyaluronic acid is the most common type of filler injected, followed by autologous fat and calcium hydroxylapatite[39]. However, visual loss has even been noted to occur secondary to platelet-rich plasma (PRP) injections and PLLA injections[43,44]. In the 4 reported cases, one woman developed painful vision loss with no light perception in the left eye after PRP injection into the left glabellar region[43]. Her fundus exam was suggestive of an embolus within the central retinal artery[43]. At her 1-month visit, she was noted to have a pale optic disc with pigment in the superior temporal region and developed a retinal detachment 8 months after her initial injection[43]. In one reported case of blindness secondary to PLLA, a patient received an injection in the left periorbital region and reported immediate pain in the left eye as well as blurring of vision[44]. On exam, the patient was found to have a decrease in vision to light perception with projection, a fixed and dilated left pupil, as well as ophthalmoplegia and ptosis[44]. While the ophthalmoplegia and ptosis improved over time, the patient’s vision still declined[44].
Although irreversible damage to the retina has been previously studied to occur within 90 min, a recent study found that retinal infarction can happen as early as 12-15 min after complete occlusion[45,46]. Given the low incidence of cases, no high-level studies currently exist that allow for a clear management recommendation for this devastating complication[47]. Ocular physical maneuvers, including compression and paracentesis, are a rapid intervention[47,48]. An ocular massage can be performed with manual firm compression to the globe in 10-20 second intervals followed by a sudden release. This compression can be performed with a Goldman lens or trans palpebral with 2 fingers[47]. The goal of these maneuvers is to lower the intraocular pressure, dilate the occluded artery and allow for the migration of the emboli to a peripheral vessel, preserving central vision[47]. Rebreathing in a brown paper bag for 10 min every 30 min can also increase CO2 and cause vasodilatation[49]. Methylprednisolone and other intravenous steroids can be used to decrease the retinal edema caused by damage to the cells[11,47]. Intraocular pressure reduction can be achieved with timolol, mannitol, and acetazolamide to restore retinal vascular flow and avoid visual loss[47]. Lastly, hyperbaric oxygen (HBOT) can be used when available, as it provides a subjective relief and can also work to improve plasma oxygen concentration and dilate the retinal arteries[47,50]. While nitroglycerin paste has been considered, questions remain on whether it is able to penetrate the deep orbital vessels[47]. One study with a rabbit eye model found that there was no improvement in perfusion with nitroglycerin paste, and the veins were also found to have a more congested appearance[51]. Additionally, dilation of the arterioles could push the product further into the smaller arterioles and capillaries[51]. The use of hyaluronidase can also be considered, especially when the filler is HA-based, within 60-90 min after visual loss[47]. While hyaluronidase has been noted to also be administered outside the 90-min window, the newest cases have not been able to show significant improvement in visual acuity.
There has been some interest in the use of HBOT in the treatment of ophthalmic artery occlusion. HBOT is defined as an intervention in which an individual “breathes near 100% oxygen intermittently while inside a treatment chamber at a pressure higher than at sea level pressure (> 1 atm)[52].” The main goal is to increase the amount of oxygen dissolved in the plasma. Increased dissolved oxygen works to improve the diffusion distance, supporting oxygen-dependent processes that do not get enough arterial blood supply[53]. One report discussed the case of a 31-year-old woman who received HA filler at the nasal dorsum and developed immediate vision loss in the right eye[54]. After being diagnosed with central retinal artery occlusion, she received a multitude of treatments including high-flow O2, ocular massage, steroids, and blood thinners[54]. She also received daily 90-min therapy of HBOT at 2.5 atmospheres absolute (ATA) or 253 kilopascals (kPa) for 3 weeks. However, the patient had no improvement in the vision in the right eye, with her visual acuity remaining at no light perception[54]. In another case, a 49-year-old woman was also found to develop a central visual defect in her right eye after the injection of poly-L-lactic acid (PLLA)[55]. Her visual acuity was found to be 20/200 in the right eye. She received ocular massage, brimonidine, and HBOT (unknown pressure) twice daily for 5 days, but did not have any improvement in her vision[55]. A 41-year-old woman who had vision loss in her right eye after a forehead filler injection received retrobulbar hyaluronidase and
The use of hyaluronidase through a retrobulbar injection is currently being debated and further studied. A 2018 case report discussed a retrobulbar injection of 450 IU of hyaluronidase, which completely restored a patient’s vision after receiving HA filler injections near the infraorbital neurovascular bundle[61]. However, this report did not document an objective visual acuity before and after hyaluronidase treatment. Another case study also showed improvement from light perception to hand motion and eventually full recovery after 2 injections of 900 IU retrobulbar hyaluronidase, 4 and 5 days after filler injection[62]. Given that these cases are anecdotal and not controlled studies, it is unclear if an improvement in vision would have occurred without retrobulbar hyaluronidase. Additionally, other reports have not shown improvement or been as definitive as to whether this treatment is a viable solution[63,64]. An animal model study showed that extravascular injection of hyaluronidase was not able to penetrate the vascular lumen or re-perfuse occluded auricular arteries[65]. This is supported by Hwang et al., in which 1000 IU of retrobulbar hyaluronidase within the ophthalmic artery 30 min after occlusion in rabbits was unable to relieve the obstruction[66]. However, in contrast, Lee et al. injected hyaluronic acid into the ophthalmic artery, confirmed ischemia with fundus photography, and then injected retrobulbar hyaluronidase[67]. While initial experiments with 1500 IU did not show any improvement, 3000 IU of hyaluronidase 5-10 min after occlusion showed an improvement in perfusion, possibly secondary to higher dosing and faster treatment[67]. Questions were raised about the methodology in the Lee et al. study regarding the lack of documented electroretinogram testing prior to treatment with hyaluronidase to detect a complete occlusion[67,68]. Hwang et al. found that the fundus photographs seemed to support a branch retinal artery occlusion rather than a complete retinal artery occlusion, possibly indicating only partial vision loss prior to treatment[68]. Additionally, the authors discuss that a dose of 3000 IU could lead to a compartment syndrome with readily available preparations (since the powdered form is not widely used in the US) and injecting within 5-10 min of occlusion would prove to be very difficult in the clinical setting[68]. Another in-vitro study showed that hyaluronidase could not cross the dural sheath of the optic nerve, which can prevent access to the central retinal artery[69]. Ugradar et al. also found that hyaluronidase was not able to reduce the particle size in a substantial manner, creating particles that were still greater than the size of the vessel[70]. These in-vitro studies highlight that physiologically, it is difficult to replicate how retrobulbar hyaluronidase can effectively relieve filler obstructions. This translates to what has been seen clinically as well, in that some patients have a full recovery of vision with retrobulbar hyaluronidase while other cases do not[29]. An additional literature review concluded that the efficacy of retrobulbar hyaluronidase was not clear and that there was not enough evidence to support retrobulbar hyaluronidase given the inherent risks[48]. This treatment has a level of evidence V, which confers a grade D recommendation from the American Society of Plastic Surgeons[48,71].
A more novel way to treat blindness from filler complications may be to consider it a stroke, because the optic nerve is part of the central nervous system. Activating a stroke protocol may be prudent. Baley-Spindel et al. noted the occlusion is likely composed of both HA gel and red thrombi; therefore, a combination of hyaluronidase and alteplase yielded the best results in clearing HA gel thrombi in their rat model[72].
Ophthalmoplegia/Double vision
While vision-threatening complications are better known, facial fillers have also been seen to cause ophthalmoplegia, as noted in Table 1. A recent study showed that 50% of patients with occlusion of the ophthalmic arfpresentation is not always as cleartery also presented with ophthalmoplegia[73]. Another study found that 15 (71%) of 21 patients over a 9-year period with artery occlusion also developed ophthalmoplegia at initial presentation, with an average of 2.8 rectus muscles involved[74]. The mechanism behind this is theorized to be from ischemia to the cranial nerves or extraocular muscles[74,75]. However, a recent report of 2 isolated ophthalmoplegia cases illustrated that this side effect could also be secondary to an inflammatory response[76].
Management of ophthalmoplegia, if a hyaluronic acid (HA) filler is used, can be performed with 1500 IU of hyaluronidase subcutaneously around the site of injection, and in the case reported by Bae et al., hospitalization was required[77]. Even though the ophthalmoplegia is resolved in some cases, sometimes the ocular misalignment persists, requiring strabismus surgery[76]. In the 2 case reports where an ischemic etiology was not suspected, one patient improved after Medrol Dosepak and aspirin 81 mg, and the other did not improve as she presented 3-4 months after the complication[76].
Soft tissue necrosis
The presentation of a patient with vascular occlusion is often characterized by pain disproportionate to the injection, along with blanching[78]. This is followed by livedo reticularis secondary to venule swelling[79,80]. However, in a clinical setting, the presentation is not always as clear, with pain often not accompanying these events[81]. For example, a net-like reddish/blue appearance may be hard to distinguish from bruising or simple erythema, but a delayed capillary refill would support ischemia. A recent study also showed that in a survey of 52 injectors, 62% had reported one or more intravascular injections, highlighting the frequency of these events[81].
The etiology of vascular complications involves arterial compromise causing tissue anoxia and progression to necrosis[15]. As the filler is being injected, it may flow in either direction in the vessel, and lead to an obstruction of the blood supply[15]. Given that fillers have inherently different properties in viscoelasticity and cohesivity, the outcomes have different severity[3,15]. Case reports with polymethylmethacrylate (PMMA) have had complications showing more dramatic skin necrosis compared to other fillers[82-84].
The glabella, nose, and nasolabial folds are at higher risk because they depend on a single arterial branch[13]. Out of all the cases of vascular necrosis, nasolabial fold injection was associated with the highest number of cases, followed by injections into the nose[85]. Nasal necrosis is likely secondary to the dorsal nasal artery having variable anatomy, occurring only 34% of the time as a pair of arteries, with other variations including a single large dorsal nasal artery or in random distribution[86]. Given the potential for embolization of facial fillers, vascular complications also have been seen to occur far from the injection area in the mid and lower face. The vascular supply of the internal nose arises from branches of the superior labial artery, the sphenopalatine artery, the posterior ethmoidal artery and the anterior ethmoidal artery[87]. After an injection of HA above the anterior nasal spine and nasal bones, a 42-year-old female developed gingival necrosis of the right upper incisor, partial lip mucosa necrosis, and an exophytic palatal lesion[87]. The gingival necrosis was likely related to embolization of the septal branches of the superior labial artery and compression of the distal arteries from the septal branch of the posterior ethmoidal artery[87]. In one author’s personal experience, she received an injection of hydroxylapatite in her left cheek, in which she noted that the needle was directed somewhat tangentially. After injection, she immediately developed blanching and mottling, which later took on a reticulated appearance due to the involvement of the infraorbital artery. She did not receive hyaluronidase at that time. The following morning, the mottling of her skin extended towards her lower eyelids and the left nasal bridge [Figure 2]. On day three, she developed pustules, necrosis of the skin, and eventually left permanent scarring [Figure 3]. Her erythema of the gums was suggestive of infraorbital artery involvement [Figure 4]. Injections into the infraorbital artery may be performed from the cheek augmentation, with the subsequent embolization extending to the facial artery below.
Figure 2. Mottling of the skin the morning after cheek injection, extending from the lower eyelid to the upper lip and part of the nose due to involvement of the infraorbital artery.
Figure 3. Formation of pustules on the nose and a full thickness defect near the nasolabial fold 3 days after injection due to involvement of the facial artery.
Skin necrosis has also been seen to occur from filler injected into the lower face. One patient, after receiving dermal fillers in the lip to correct an atrophic scar, presented with pain and blanching of the upper lip with a blue tinge 2 hours after her initial injection[88]. The authors did note that the necrosis may have been secondary to an alteration of the blood vessel course from scarring already present in the area[88]. Injections in the lower lip may need to be cautious of the inferior labial artery, which has been studied to have variation in its dominant arterial sources [Figure 5][89].
Figure 5. Representative figure of the main arteries of the lower face and chin, with depiction of the variation of arterial sources of the inferior labial arteries. Figure reproduced from Tansatit et al.[89].
Skin necrosis with chin injections has also been reported[90,91]. In one case, the patient developed numbness on the right side of her tongue during an injection of her chin and was found to have an obstruction of her deep lingual artery[90]. In another case, after chin augmentation injection, a patient developed pain with swallowing as well as livedo reticularis and mottling from the mental crease to the upper cervical area
If vascular occlusion is suspected with pallor and blanching, the injection must be stopped immediately[13,78,92]. Aspiration of the product should be attempted before withdrawing the needle[13,78]. With the goal of improving blood flow, warm compresses and a massage of the area should be done[13,76,90]. Regardless of filler type, hyaluronidase should be injected as it has been shown to reduce edema[93,94]. Aspirin should also be started to limit clot propagation and platelet activation and be given with an antacid[13,92]. In one case report, low level light therapy (LLLT) was used with the intention of reducing pain and inflammation, and improving tissue repair and regeneration[77]. The goal of LLLT is to use photons at a non-thermal value to change biological activity[95]. Through the enhancement of specific enzyme activity, LLLT has been shown to activate intracellular signaling pathways and transcription factors involved with cell proliferation, survival, and tissue repair[95]. Nishioka et al. have also shown that LLLT therapy increases skin flap viability in rats, with the percentage of necrosis area of the flap decreasing in the LLLT group[96]. Sildenafil, tadalafil, and vardenafil can also be used to relax smooth muscles, dilate blood vessels, and increase blood flow[97]. The use of nitroglycerin has not been fully defined. Although nitroglycerin paste has innately vasodilatory properties, an animal study showed no improvement in perfusion and raised the question of whether arterioles dilation could cause further propagation of filler and worsen the ischemia[51]. Given its inherent risks of headaches and hypotension, van Loghem et al. did not make a clear recommendation on whether to incorporate nitroglycerin paste[13].
Hyperbaric oxygen has also been reported to be an effective adjunct treatment for vascular occlusion. A 32-year-old female who developed vascular occlusion after HA filler on her nose received 1 month of biweekly 90 min HBOT sessions at 2.4 ATA, after which her nose showed improvement in vascularization[98]. Another report also remarked on a 37-year-old female who developed ischemic changes to her face after an injection on her proximal temple[99]. After receiving 6 treatments twice daily (2 treatments at 3.0 ATA followed by 4 treatments at 2.4 ATA), the patient showed improvement in ischemic discoloration[99]. Two cases, one involving a 46-year-old man who received poly-methylmethacrylate and calcium hydroxylapatite and a 40-year-old white woman who was treated with hyaluronic acid, showed improvement in their skin necrosis after HBOT[94]. One treatment lasted 14 days, while the other lasted 2 days[94]. Thus, while HBOT remains an inconclusive treatment for vascular occlusion of the ophthalmic and retinal artery, it has been shown to potentially have more benefit in skin necrosis treatment.
Recently, a new protocol has been released in which high dose pulsed hyaluronidase is used for vascular adverse events. For low volume events (0.1 mL or less of HA filler), 450 IU of hyaluronidase is used in a single area, for an area half of an upper lip[100]. If the nose has involvement, 900 IU of hyaluronidase should be used[100]. The dosing should also be hourly rather than the traditional daily dosing to maintain high concentrations in the ischemic zone[100]. The injections should be given at injection sites, and if there are distal sites that appear ischemic, one should consider injecting distal sites of ischemia[15]. Delorenzi et al. have remarked that hyaluronidase is able to diffuse through an arterial wall, so the most important area of injection would be areas that show ischemia[15]. However, the article did comment on a case in which the patient did not initially improve with hyaluronidase injection into the ischemic tissue, but showed improvement after hyaluronidase was injected into the affected artery[15]. Thus, further studies may be needed to elucidate the most effective area for hyaluronidase injection immediately after vascular occlusion.
Cerebral infarction
Filler injections can lead to very severe complications. Cerebral infarction secondary to vascular occlusion has been noted as a complication of filler injections. A review article of 44 cases showed that 8 patients (18.2%) had CNS involvement, including upper limb weakness, acute infarction, or hemorrhage[29]. A 20-year-old female who presented with non-improving vision loss in her right eye was found to have multifocal infarcts in her parietal lobes[101]. This patient received injections in the glabella, an area known to cause combined ophthalmic and cerebral complications[101]. Anatomically, the supratrochlear and supraorbital arteries were injected with enough force that the filler traveled retrogradely to enter the cerebral circulation via the Circle of Willis[101]. Another study involved a 39-year-old female who presented with vision loss in the left eye after filler injection into the glabella[102]. This vision loss was concurrent with ptosis and total ophthalmoplegia[102]. The next day, an MRI found several cerebral infarctions that were embolic in nature[102]. After one week, the infarction transformed into a parenchymal hematoma, after which the patient received methylprednisolone[102]. While her limb weakness improved, she continued to have a right arm monoparesis[102]. Another case involved a 40-year-old female who developed a cerebral infarction after nasal augmentation[103]. With a GCS of 4 and on mechanical ventilation, the patient developed gastric ulceration, pulmonary infection, respiratory failure, and cerebral herniation, dying 6 days after the filler injection[103]. For this patient, the theory was that there were several small HA particles in the capillaries from this filler injection[103]. The authors recommended immediate thrombolysis within the 12-h window of functional impairment (90 min for concomitant ophthalmic arterial occlusion) and consideration of decompressive craniectomy[103].
PREVENTION
Given the severity of these intravascular complications, prevention is key in delivering high-quality patient care. Knowledge of various injection techniques and the relatively new utilization of imaging have been studied in the prevention of these outcomes.
Injection techniques
There are several injection techniques that have been studied to help prevent vascular occlusions. Aspiration before injection (with an unprimed needle, especially if the filler is thick or sticky) and using lower volumes of the product (0.1 mL) can help indicate that the needle has not entered a blood vessel and reduce the severity of complications if a blood vessel is entered[7,17]. Sometimes aspiration can give you a false sense of security, as the filler may be too thick to detect reflux of blood when in a vessel. If injecting in the areas of the supratrochlear artery, supraorbital artery, and dorsal nasal artery, compression of the vessel pathway can help prevent retrograde flow[104]. The use of a reversible HA filler will allow for treatment with hyaluronidase if a reversal of vascular occlusion is needed[7]. Given that the pressure of the filler injection can cause retrograde flow, injecting at a slow pace can help in preventing complications[7,105].
Needle size has also been seen to have an impact on vascular obstruction. While a smaller needle may improve the precision of the injection, it may increase the likelihood of penetrating the vessel wall rather than a larger bore needle which would roll on the side of the artery[15,104]. Additionally, more pressure is required to inject through a smaller bore needle which could lead to more pressure into a vessel, should vasculature be entered. A recent study also found that the use of microcannulas had 77% lower odds of occlusion compared to needle injections, due to the fact that blunt tips could avoid piercing vessel walls[106]. However, small bore microcannulas still have the potential to penetrate the facial artery with a small amount of force (0.23 kg for a 27G cannula)[107]. Epinephrine may also help with vasoconstriction, but may make it more difficult to distinguish an early manifestation of necrosis[104]. Thus, administering local anesthesia without epinephrine may be considered[104,108].
Imaging
Mapping vasculature to prevent intravascular filler injection
Various studies have now started incorporating ultrasound as a method to map vasculature and reduce the incidence of vascular complications seen with filler injections. Doppler ultrasound has been seen to detect anatomy, such as the facial artery lateral to the nasolabial fold, along with its different anatomical variations[109]. One other study commented that although ultrasound may be difficult to manage in conjunction with injection, a new technique combining 3D time of flight magnetic resonance angiography (3D-TOF MOTSA MRA) and infrared (IR) facial heating could visualize the following arteries: facial, angular, superior labial, inferior labial, lateral nasal, dorsal nasal, supratrochlear, supraorbital, and superficial temporal[110]. These images could be acquired on a 1.5 or 3 Tesla (T) system with a head coil, with an additional surface coil on top, to improve signal reception[110]. The authors showed that these MRA images could be projected on the patient’s face before injection, albeit without the same 3D depth aspect[110]. The main benefit of this method was visualization of the facial arteries in a non-invasive and contrast-free manner[110]. However, the cost of MRI in these individual countries may need to be considered before the widespread adoption of this technology. Our team has also looked at the utilization of 7T MRI and shown its ability to depict small orbital and eyelid structures, as well as the orbital branch of the infraorbital artery (article in press).
The use of ultrasound has been adopted at various points in the filler injection procedure. Firstly, ultrasound has successfully determined that the needle or cannula is positioned in the correct plane and not within or near a vessel[111]. Schelke et al. discussed how the use of doppler ultrasound can help distinguish vessels as well as the direction of blood flow [Figure 6][112]. Rocha et al. recommended that once the needle or cannula is determined to be in the correct location, the filler can be injected without aspiration[111]. After the injection of filler, doppler ultrasound can again be used to confirm the vascularization of the area[111]. As seen in Figure 7, the placement of filler deposits, such as hyaluronic acid, can be distinctly visualized on ultrasound to confirm correct depth and placement.
Figure 6. Localization of the artery with ultrasound. Figure reproduced with permission from Schelke et al.[112].
Figure 7. Multiple deposits of hyaluronic acid filler, as represented by the two anechoic deposits and one hypoechoic deposit. Figure reproduced with permission from Schelke et al.[112].
Diagnosing areas of ischemia from filler complications
Imaging can also be used in the early diagnosis and treatment of filler complications. Another study evaluating laser doppler imaging (LDI) found that LDI was able to accurately delineate a hypo-perfused area to help target hyaluronidase treatment[113]. Color Doppler flow imaging (CDFI) was able to successfully detect retinal artery occlusions and ophthalmic artery caused by filler injections by showing decreased retrobulbar blood flow[114]. Doppler ultrasound may be able to be used to detect the lack of perfusion, and in rare cases, an ischemic vessel may be able to be injected with hyaluronidase under ultrasound guidance. Schelke et al. reported on a case of how, upon crusting under the lip after upper lip augmentation, 150 U of hyaluronidase was injected with ultrasound guidance[112]. A case in which ultrasound was used to inject hyaluronidase into a visualized filler deposit is seen in Figure 8. In another case, after a patient presented with mottling of her chin following filler injection, ultrasound was used as guidance for hyaluronidase injection [Figure 9]. Ultrasound imaging appears to be valuable in helping the injector decide where to place the hyaluronidase. However, this may be very difficult to do, and it is unclear how much an ultrasound may help or distract from the process of trying to cannulate a vessel.
Figure 8. Needle inserted into filler deposit under ultrasound guidance. Figure reproduced with permission from Schelke et al.[112].
Figure 9. Using ultrasound (Clarius Portable Ultrasound L20) in a clinical setting to localize filler placement causing skin ischemia and injecting hyaluronidase (see Supplementary Video 1 and Supplementary Video 2)
Magnetic resonance imaging (MRI) has also been studied as the primary mechanism to evaluate for infarctions after ischemic complications. Many of the case reports that were reviewed showed that an MRI or magnetic resonance angiography (MRA) was used to evaluate intracranial infarctions and embolisms[101,102,115]. Additionally, the use of MRI is finding an increasing role in the detection of other non-ischemic complications associated with fillers such as inflammation, foreign body granulomas, and filler migrations, which we will expand upon in another review paper[116]. While the use of imaging in this field is still relatively recent, it represents an area for further development and utilization.
REVERSIBILITY
Understanding the reversibility of fillers is important, should an ischemic or non-ischemic complication occur. Fat and calcium hydroxylapatite are not reversible, although some reports have seen improvement in calcium hydroxylapatite injections with simple saline diffusion[24], hyaluronidase[25], sodium thiosulfate[26], steroids[27], and 5-fluorouracil[28] injections. Additionally, many of the new hyaluronic acid gel fillers require high doses of hyaluronidase and multiple injections to reverse. For example, Restylane-L and Restylane-Lyft are easy to dissolve, whereas the Vycross products, RHA3-4, and the newer Restylanes are much more difficult to reverse. Refer to previous publications[21-23] and upcoming papers to remain updated on all the different filler products.
CONCLUSION
As filler injections become more widespread, it is important to be aware of and know how to manage the devastating ischemic complications that can occur. Most of these ischemic complications occur with pain disproportionate to the injection and with a sudden change in vision or blanching or dusking of the skin. Ischemic complications may also present in a more delayed fashion in the first day or two after injection with mottled reticular-appearing skin. Treatment of ischemic complications begins with early identification of the ischemia, including being aware of cerebrovascular events, and early treatment of ophthalmic artery occlusions within 90 min. Aspirin and other anticoagulation can be used, but the main tool is early delivery of hyaluronidase of 450-3000 units in areas that can accommodate that volume, spread over multiple boluses, depending on the area and severity of ischemia. Cannulating an artery, perhaps with image and doppler guidance, for hyaluronidase injection would be ideal, but this is very challenging. For blindness, activating a stroke protocol at a nearby hospital may even be considered to treat the red thrombi component of arterial occlusions. Warm compresses and ocular massage (for ocular ischemia), hyperbaric oxygen therapy or low-level light therapy (for soft tissue ischemia) can also be considered. Nitroglycerin paste is controversial. Hyperbaric oxygen can be considered to help salvage marginal tissue that may otherwise become necrotic. No panacea exists except for prevention. Due to simple mathematics, occlusion may not be entirely avoidable if one is injected enough, despite one’s best efforts. However, minimizing the incidence of these complications requires knowledge of the local anatomy, filler properties (reversible non-permanent filler is safer), and utilizing the safest injection techniques. New advances in the field include utilizing imaging to help avoid and diagnose intravascular injection. Higher concentrations of hyaluronidase may also be required to reverse the thicker and newer hyaluronic acid gel fillers.
DECLARATIONS
Authors’ contributionsMade substantial contributions to conception and design of the study and performed data analysis and interpretation: Mehta P, Kaplan JB, Zhang-Nunes S
Availability of data and materialsNot applicable.
Financial support and sponsorshipThis work was supported by an unrestricted Grant to the Department of Ophthalmology from Research to Prevent Blindness, New York, NY and the Keck School of Medicine Dean’s Research Scholar Award.
Conflicts of interestAll authors declared that there are no related conflicts of interest.
Ethical approval and consent to participateNot applicable.
Consent for publicationNot applicable.
Copyright© The Author(s) 2022.
Supplementary MaterialsREFERENCES
1. ISAPS International Survey on Aesthetic/Cosmetic Procedures. Available from: https://www.isaps.org/wp-content/uploads/2020/12/Global-Survey-2019.pdf [Last accessed on 1 Sep 2022].
2. FDA Executive Summary General Issues Panel Meeting on Dermal Fillers. Prepared for the meeting of the general and plastic surgery devices advisory panel. Available from: https://www.fda.gov/media/146870/download [Last accessed on 1 Sep 2022].
3. Heitmiller K, Ring C, Saedi N. Rheologic properties of soft tissue fillers and implications for clinical use. J Cosmet Dermatol 2021;20:28-34.
4. Murthy R, Roos JCP, Goldberg RA. Periocular hyaluronic acid fillers: applications, implications, complications. Curr Opin Ophthalmol 2019;30:395-400.
5. Beleznay K, Carruthers JDA, Humphrey S, Carruthers A, Jones D. Update on avoiding and treating blindness from fillers: a recent review of the world literature. Aesthet Surg J 2019;39:662-74.
6. Kim A, Kim SH, Kim HJ, Yang HK, Hwang JM, Kim JS. Ophthalmoplegia as a complication of cosmetic facial filler injection. Acta Ophthalmol 2016;94:e377-9.
7. Beleznay K, Humphrey S, Carruthers JDA, Carruthers A. Vascular compromise from soft tissue augmentation: experience with 12 cases and recommendations for optimal outcomes. J Clin Aesthet Dermatol 2014;7:37-43.
8. Chatrath V, Banerjee PS, Goodman GJ, Rahman E. Soft-tissue filler-associated blindness: a systematic review of case reports and case series. Plast Reconstr Surg Glob Open 2019;7:e2173.
9. Kim YJ, Choi KS. Bilateral blindness after filler injection. Plast Reconstr Surg 2013;131:298e-9e.
10. Oranges CM, Brucato D, Schaefer DJ, Kalbermatten DF, Harder Y. Complications of nonpermanent facial fillers: a systematic review. Plast Reconstr Surg Glob Open 2021;9:e3851.
11. Beleznay K, Carruthers JD, Humphrey S, Jones D. Avoiding and treating blindness from fillers: a review of the world literature. Dermatol Surg 2015;41:1097-117.
12. Rohrich RJ, Bartlett EL, Dayan E. Practical approach and safety of hyaluronic acid fillers. Plast Reconstr Surg Glob Open 2019;7:e2172.
13. van Loghem J, Funt D, Pavicic T, et al. Managing intravascular complications following treatment with calcium hydroxylapatite: an expert consensus. J Cosmet Dermatol 2020;19:2845-58.
14. Goodman GJ, Magnusson MR, Callan P, et al. A consensus on minimizing the risk of hyaluronic acid embolic visual loss and suggestions for immediate bedside management. Aesthet Surg J 2020;40:1009-21.
15. DeLorenzi C. Complications of injectable fillers, part 2: vascular complications. Aesthet Surg J 2014;34:584-600.
16. Ten B, Kara T, Kaya Tİ, et al. Evaluation of facial artery course variations and depth by Doppler ultrasonography. J Cosmet Dermatol 2021;20:2247-58.
17. Tseng FW, Bommareddy K, Frank K, et al. Descriptive analysis of 213 positive blood aspiration cases when injecting facial soft tissue fillers. Aesthet Surg J 2021;41:616-24.
18. Lee JG, Yang HM, Hu KS, et al. Frontal branch of the superficial temporal artery: anatomical study and clinical implications regarding injectable treatments. Surg Radiol Anat 2015;37:61-8.
19. Maio M, DeBoulle K, Braz A, Rohrich RJ; Alliance for the Future of Aesthetics Consensus Committee. Facial assessment and injection guide for botulinum toxin and injectable hyaluronic acid fillers: focus on the midface. Plast Reconstr Surg 2017;140:540e-50e.
20. Hufschmidt K, Bronsard N, Foissac R, et al. The infraorbital artery: clinical relevance in esthetic medicine and identification of danger zones of the midface. J Plast Reconstr Aesthet Surg 2019;72:131-6.
21. Zhang-Nunes S, Ryu C, Cahill K, et al. Prospective in vivo evaluation of three different hyaluronic acid gels to varying doses of hyaluronidase with long-term follow-up. J Plast Reconstr Aesthet Surg 2021;74:874-80.
22. Ryu C, Lu JE, Zhang-Nunes S. Response of twelve different hyaluronic acid gels to varying doses of recombinant human hyaluronidase. J Plast Reconstr Aesthet Surg 2021;74:881-9.
23. Zhang-Nunes S, Mehta P, Ryu C, Zhou Q. Response of five different hyaluronic acid gels to varying doses of recombinant human hyaluronidase. In: American Society of Ophthalmic Plastic & Reconstructive Surgery Spring Meeting 2022. Available from: https://asoprsconf.memberclicks.net/assets/docs/ASOPRS_2022_Spring_Program.pdf [Last accessed on 1 Sep 2022].
24. Vrcek IM, Malouf P, Gilliland GD. A novel solution for superficially placed calcium hydroxylapatite (Radiesse) in the inferior eyelid. Orbit 2012;31:431-2.
25. Vu PQ, Grob SR, Tao JP. Light perception vision recovery after treatment for calcium hydroxylapatite cosmetic filler-induced blindness. Ophthalmic Plast Reconstr Surg 2018;34:e189-92.
26. Danysz W, Nowag B, Hengl T, et al. Can sodium thiosulfate act as a reversal agent for calcium hydroxylapatite filler? Clin Cosmet Investig Dermatol 2020;13:1059-73.
27. Rivkin AZ. Volume correction in the aging hand: role of dermal fillers. Clin Cosmet Investig Dermatol 2016;9:225-32.
28. Aguilera SB, Aristizabal M, Reed A. Successful treatment of calcium hydroxylapatite nodules with intralesional 5-fluorouracil, dexamethasone, and triamcinolone. J Drugs Dermatol 2016;15:1142-3.
29. Kapoor KM, Kapoor P, Heydenrych I, Bertossi D. Vision loss associated with hyaluronic acid fillers: a systematic review of literature. Aesthetic Plast Surg 2020;44:929-44.
30. Sito G, Manzoni V, Sommariva R. Vascular complications after facial filler injection: a literature review and meta-analysis. J Clin Aesthet Dermatol 2019;12:E65-E72.
31. Samizadeh S, Pirayesh A, Bertossi D. Anatomical variations in the course of labial arteries: a literature review. Aesthet Surg J 2019;39:1225-35.
32. Toure G, Nguyen TM, Vlavonou S, Ndiaye MM. Transverse facial artery: its role in blindness after cosmetic filler and botulinum toxin injections. J Plast Reconstr Aesthet Surg 2021;74:1862-9.
33. Tansatit T, Phumyoo T, Jitaree B, Sawatwong W, Sahraoui YME. Investigation of the presence and variation of the ascending mental artery: Conventional dissections and ultrasonographic study. J Cosmet Dermatol 2019;18:1821-9.
34. Myung Y, Yim S, Jeong JH, et al. The classification and prognosis of periocular complications related to blindness following cosmetic filler injection. Plast Reconstr Surg 2017;140:61-4.
35. Coleman SR. Avoidance of arterial occlusion from injection of soft tissue fillers. Aesthet Surg J 2002;22:555-7.
36. Carruthers JDA, Fagien S, Rohrich RJ, Weinkle S, Carruthers A. Blindness caused by cosmetic filler injection: a review of cause and therapy. Plast Reconstr Surg 2014;134:1197-201.
37. Cho KH, Dalla Pozza E, Toth G, Bassiri Gharb B, Zins JE. Pathophysiology study of filler-induced blindness. Aesthet Surg J 2019;39:96-106.
39. Sorensen EP, Council ML. Update in soft-tissue filler-associated blindness. Dermatol Surg 2020;46:671-7.
40. Ugradar S, Diniz S, Hoenig J, Goldberg RA. Generation of filler emboli as a mechanism for filler-related blindness. Dermatol Surg 2021;47:235-7.
41. Ramesh S, Le A, Katsev B, Ugradar S. The force required to inject a column of filler through facial arteries. Dermatol Surg 2020;46:e32-7.
42. Khan TT, Colon-Acevedo B, Mettu P, DeLorenzi C, Woodward JA. An anatomical analysis of the supratrochlear artery: considerations in facial filler injections and preventing vision loss. Aesthet Surg J 2017;37:203-8.
43. Karam EZ, Gan A, Muci Mendoza R, Martinez E, Perez E. Visual loss after platelet-rich plasma injection into the face. Neuroophthalmology 2020;44:371-8.
44. Roberts SA, Arthurs BP. Severe visual loss and orbital infarction following periorbital aesthetic poly-(L)-lactic acid (PLLA) injection. Ophthalmic Plast Reconstr Surg 2012;28:e68-70.
45. Hayreh SS, Weingeist TA. Experimental occlusion of the central artery of the retina. IV: retinal tolerance time to acute ischaemia. Br J Ophthalmol 1980;64:818-25.
46. Tobalem S, Schutz JS, Chronopoulos A. Central retinal artery occlusion - rethinking retinal survival time. BMC Ophthalmol 2018;18:101.
47. Graue G, Ochoa Araujo DA, Plata Palazuelos C, et al. The M.A.STE.R.S algorithm for acute visual loss management after facial filler injection. J Cosmet Dermatol 2020;19:2859-66.
48. Paap MK, Milman T, Ugradar S, Goldberg R, Silkiss RZ. Examining the role of retrobulbar hyaluronidase in reversing filler-induced blindness: a systematic Review. Ophthalmic Plast Reconstr Surg 2020;36:231-8.
49. Heydenrych I, De Boulle K, Kapoor KM, Bertossi D. The 10-point plan 2021: updated concepts for improved procedural safety during facial filler treatments. Clin Cosmet Investig Dermatol 2021;14:779-814.
50. Butler FK Jr, Hagan C, Murphy-Lavoie H. Hyperbaric oxygen therapy and the eye. Undersea Hyperb Med 2008;35:333-87.
51. Hwang CJ, Morgan PV, Pimentel A, Sayre JW, Goldberg RA, Duckwiler G. Rethinking the role of nitroglycerin ointment in ischemic vascular filler complications: an animal model with ICG imaging. Ophthalmic Plast Reconstr Surg 2016;32:118-22.
53. Uittenbogaard D, Lansdorp CA, Bauland CG, Boonstra O. Hyperbaric oxygen therapy for dermal ischemia after dermal filler injection with calcium hydroxylapatite: a case report. Undersea Hyperb Med 2019;46:207-10.
54. Hung JH, Wang CT, Lee CN, Shieh SJ. Unilateral vision loss after hyaluronic acid injection: a case report. Ann Plast Surg 2021;86:S127-31.
55. Wu CW, Wu HJ. Retinal artery occlusion following cosmetic injection of poly-L-lactic acid. Taiwan J Ophthalmol 2021;11:317-20.
56. Hu XZ, Hu JY, Wu PS, Yu SB, Kikkawa DO, Lu W. Posterior ciliary artery occlusion caused by hyaluronic acid injections into the forehead: a case report. Medicine (Baltimore) 2016;95:e3124.
57. Celebi ARC, Kilavuzoglu AE, Altiparmak UE, Cosar CB, Ozkiris A. Hyperbaric oxygen for the treatment of the rare combination of central retinal vein occlusion and cilioretinal artery occlusion. Diving Hyperb Med 2016;46:50-3.
58. Kim YS, Nam MS, Park EJ, et al. The effect of adjunctive hyperbaric oxygen therapy in patients with central retinal artery occlusion. Undersea Hyperb Med 2020;47:57-64.
59. Kim SH, Cha YS, Lee Y, Kim H, Yoon IN. Successful treatment of central retinal artery occlusion using hyperbaric oxygen therapy. Clin Exp Emerg Med 2018;5:278-81.
60. Lemos JA, Teixeira C, Carvalho R, Fernandes T. Combined central retinal artery and vein occlusion associated with factor V leiden mutation and treated with hyperbaric oxygen. Case Rep Ophthalmol 2015;6:462-8.
61. Chesnut C. Restoration of visual loss with retrobulbar hyaluronidase injection after hyaluronic acid filler. Dermatol Surg 2018;44:435-7.
62. Wibowo A, Kapoor KM, Philipp-Dormston WG. Reversal of post-filler vision loss and skin ischaemia with high-dose pulsed hyaluronidase injections. Aesthetic Plast Surg 2019;43:1337-44.
63. Thanasarnaksorn W, Cotofana S, Rudolph C, Kraisak P, Chanasumon N, Suwanchinda A. Severe vision loss caused by cosmetic filler augmentation: case series with review of cause and therapy. J Cosmet Dermatol 2018;17:712-8.
64. Zhu GZ, Sun ZS, Liao WX, et al. Efficacy of retrobulbar hyaluronidase injection for vision loss resulting from hyaluronic acid filler embolization. Aesthet Surg J 2017;38:12-22.
65. Zhang L, Feng X, Shi H, Wu WTL, Wu S. Blindness after facial filler injections: the role of extravascular hyaluronidase on intravascular hyaluronic acid embolism in the rabbit experimental model. Aesthet Surg J 2020;40:319-26.
66. Hwang CJ, Mustak H, Gupta AA, Ramos RM, Goldberg RA, Duckwiler GR. Role of retrobulbar hyaluronidase in filler-associated blindness: evaluation of fundus perfusion and electroretinogram readings in an animal model. Ophthalmic Plast Reconstr Surg 2019;35:33-7.
67. Lee W, Oh W, Ko HS, Lee SY, Kim KW, Yang EJ. Effectiveness of retrobulbar hyaluronidase injection in an iatrogenic blindness rabbit model using hyaluronic acid filler injection. Plast Reconstr Surg 2019;144:137-43.
68. Hwang CJ, Perry JD. Effectiveness of retrobulbar hyaluronidase injection in an iatrogenic blindness rabbit model using hyaluronic acid filler injection. Plast Reconstr Surg 2020;145:658e-60e.
69. Paap MK, Milman T, Ugradar S, Silkiss RZ. Assessing retrobulbar hyaluronidase as a treatment for filler-induced blindness in a cadaver model. Plast Reconstr Surg 2019;144:315-20.
70. Ugradar S. Quantifying the digestion of cross-linked hyaluronic acid fillers with hyaluronidase. Dermatol Surg 2021;47:1233-6.
71. Burns PB, Rohrich RJ, Chung KC. The levels of evidence and their role in evidence-based medicine. Plast Reconstr Surg 2011;128:305-10.
72. Baley-Spindel I, Villaseñor-Villalpando E, Márquez-Espriella C, Rivera-Salgado MI, Dávila-Díaz R. Perivascular hyaluronidase with alteplase as treatment for hyaluronic acid thrombosis. Aesthet Surg J 2020;40:551-9.
73. Park KH, Kim YK, Woo SJ, et al. Korean Retina Society. Iatrogenic occlusion of the ophthalmic artery after cosmetic facial filler injections: a national survey by the Korean Retina Society. JAMA Ophthalmol 2014;132:714-23.
74. Yang HK, Lee Y, Woo SJ, Park KH, Kim JS, Hwang JM. Natural course of ophthalmoplegia after iatrogenic ophthalmic artery occlusion caused by cosmetic filler injections. Plast Reconstr Surg 2019;144:28e-34e.
75. Zimmerman CF, Van Patters PD, Golnik KC, Kopitnik TA, Anand R. Orbital infarction syndrome after surgery for intracranial aneurysms. Ophthalmology 1995;102:594-8.
76. Downie EM, Chen Y, Lucarelli MJ, Burkat CN. Isolated ophthalmoplegia following filler injections to the upper face. Ophthalmic Plast Reconstr Surg 2020;36:e152-4.
77. Bae IH, Kim MS, Choi H, Na CH, Shin BS. Ischemic oculomotor nerve palsy due to hyaluronic acid filler injection. J Cosmet Dermatol 2018;17:1016-8.
78. Sclafani AP, Fagien S. Treatment of injectable soft tissue filler complications. Dermatol Surg 2009;35 Suppl 2:1672-80.
79. Loh KTD, Phoon YS, Phua V, Kapoor KM. Successfully managing impending skin necrosis following hyaluronic acid filler injection, using high-dose pulsed hyaluronidase. Plast Reconstr Surg Glob Open 2018;6:e1639.
80. Boulle K, Heydenrych I. Patient factors influencing dermal filler complications: prevention, assessment, and treatment. Clin Cosmet Investig Dermatol 2015;8:205-14.
81. Goodman GJ, Roberts S, Callan P. Experience and management of intravascular injection with facial fillers: results of a multinational survey of experienced injectors. Aesthetic Plast Surg 2016;40:549-55.
82. Silva MT, Curi AL. Blindness and total ophthalmoplegia after aesthetic polymethylmethacrylate injection: case report. Arq Neuropsiquiatr 2004;62:873-4.
83. Figueiredo JC, Naufal RR, Zampar AG, Mélega JM. Expanded median forehead flap and abbé flap for nasal and upper lip reconstruction after complications of polymethylmethacrylate. Aesthetic Plast Surg 2010;34:385-7.
84. Cohen JL. Understanding, avoiding, and managing dermal filler complications. Dermatol Surg 2008;34 Suppl 1:S92-9.
85. Ortiz AE, Ahluwalia J, Song SS, Avram MM. Analysis of U.S. food and drug administration data on soft-tissue filler complications. Dermatol Surg 2020;46:958-61.
86. Tansatit T, Apinuntrum P, Phetudom T. Facing the worst risk: confronting the dorsal nasal artery, implication for non-surgical procedures of nasal augmentation. Aesthetic Plast Surg 2017;41:191-8.
87. Rauso R, Bove P, Rugge L, Chirico F. Unusual intraoral necrosis after hyaluronic acid injections. Dermatol Surg 2021;47:1158-60.
88. Chuchvara N, Alamgir M, John AM, Rao B. Dermal filler-induced vascular occlusion successfully treated with tadalafil, hyaluronidase, and aspirin. Dermatol Surg 2021;47:1160-2.
89. Tansatit T, Phumyoo T, MCCabe H, Jitaree B. Translucent and ultrasonographic studies of the inferior labial artery for improvement of filler injection techniques. Plast Reconstr Surg Glob Open 2019;7:e2399.
90. Wang Q, Zhao Y, Li H, Li P, Wang J. Vascular complications after chin augmentation using hyaluronic acid. Aesthetic Plast Surg 2018;42:553-9.
91. Fang M, Rahman E, Kapoor KM. Managing complications of submental artery involvement after hyaluronic acid filler injection in chin region. Plast Reconstr Surg Glob Open 2018;6:e1789.
92. Murray G, Convery C, Walker L, Davies E. Guideline for the management of hyaluronic acid filler-induced vascular occlusion. J Clin Aesthet Dermatol 2021;14:E61-9.
93. Dayan SH. Complications from toxins and fillers in the dermatology clinic: recognition, prevention, and treatment. Facial Plast Surg Clin North Am 2013;21:663-73.
94. Darling MD, Peterson JD, Fabi SG. Impending necrosis after injection of hyaluronic acid and calcium hydroxylapatite fillers: report of 2 cases treated with hyperbaric oxygen therapy. Dermatol Surg 2014;40:1049-52.
95. Avci P, Gupta A, Sadasivam M, Vecchio D, Pam Z, Pam N, Hamblin MR. Low-level laser (light) therapy (LLLT) in skin: stimulating, healing, restoring. Semin Cutan Med Surg 2013;32:41-52.
96. Nishioka MA, Pinfildi CE, Sheliga TR, Arias VE, Gomes HC, Ferreira LM. LED (660 nm) and laser (670 nm) use on skin flap viability: angiogenesis and mast cells on transition line. Lasers Med Sci 2012;27:1045-50.
97. Beer K, Downie J, Beer J. A treatment protocol for vascular occlusion from particulate soft tissue augmentation. J Clin Aesthet Dermatol 2012;5:44-7.
98. Oley MH, Oley MC, Mawu FO, Aling DMR, Faruk M. Hyperbaric oxygen therapy in managing minimally invasive aesthetic procedure complications: a report of three cases. Clin Cosmet Investig Dermatol 2022;15:63-8.
99. Henderson R, Reilly DA, Cooper JS. Hyperbaric oxygen for ischemia due to injection of cosmetic fillers: case report and issues. Plast Reconstr Surg Glob Open 2018;6:e1618.
100. DeLorenzi C. New high dose pulsed hyaluronidase protocol for hyaluronic acid filler vascular adverse events. Aesthet Surg J 2017;37:814-25.
101. Ansari ZA, Choi CJ, Rong AJ, Erickson BP, Tse DT. Ocular and cerebral infarction from periocular filler injection. Orbit 2019;38:322-4.
102. Lee JS, Kim JY, Woo SJ. Unilateral blindness with bilateral brain infarction after cosmetic facial filler injection. J Neuroophthalmol 2021;41:e566-71.
103. Yang Q, Lu B, Guo N, et al. Fatal cerebral infarction and ophthalmic artery occlusion after nasal augmentation with hyaluronic acid-a case report and review of literature. Aesthetic Plast Surg 2020;44:543-8.
104. Lee W, Koh IS, Oh W, Yang EJ. Ocular complications of soft tissue filler injections: A review of literature. J Cosmet Dermatol 2020;19:772-81.
105. Ozturk CN, Li Y, Tung R, Parker L, Piliang MP, Zins JE. Complications following injection of soft-tissue fillers. Aesthet Surg J 2013;33:862-77.
106. Alam M, Kakar R, Dover JS, et al. Rates of vascular occlusion associated with using needles vs. cannulas for filler injection. JAMA Dermatol 2021;157:174-80.
107. Ugradar S, Hoenig J. Measurement of the force required by blunt-tipped microcannulas to perforate the facial artery. Ophthalmic Plast Reconstr Surg 2019;35:444-6.
108. Halepas S, Peters SM, Goldsmith JL, Ferneini EM. Vascular compromise after soft tissue facial fillers: case report and review of current treatment protocols. J Oral Maxillofac Surg 2020;78:440-5.
109. Lee W, Kim JS, Moon HJ, Yang EJ. A safe doppler ultrasound-guided method for nasolabial fold correction with hyaluronic acid filler. Aesthet Surg J 2021;41:NP486-92.
110. Mespreuve M, Waked K, Hendrickx B. Visualization techniques of the facial arteries. J Cosmet Dermatol 2021;20:386-90.
111. Rocha PS, Guerra TA, Teixeira DA. Description of a safe doppler ultrasound-guided technique for hyaluronic acid filler in the face-A method to avoid adverse vascular events. J Cosmet Dermatol 2022;21:2783-7.
112. Schelke LW, Decates TS, Velthuis PJ. Ultrasound to improve the safety of hyaluronic acid filler treatments. J Cosmet Dermatol 2018;17:1019-24.
113. Lee AL, Chen YF, Yao WT, et al. Laser doppler imaging for treating vascular complications from procedures involving dermal fillers: case series and literature review. Diagnostics (Basel) 2021;11:1640.
114. Huang P, Liu A, Ren H, Xue K. Color doppler flow imaging of retrobulbar ocular blood flow changes in retinal artery occlusions caused by cosmetic facial filler injections. Ophthalmic Plast Reconstr Surg 2019;35:227-31.
115. AlGhadeer H, Talea M, Al-Muhaylib A, AlSheikh O, Elkhamary SM. Acute unilateral vision loss and bilateral cerebral infarction following cosmetic filler injection. Orbit 2021; doi: 10.1080/01676830.2021.1974056.
116. Mundada P, Kohler R, Boudabbous S, Toutous Trellu L, Platon A, Becker M. Injectable facial fillers: imaging features, complications, and diagnostic pitfalls at MRI and PET CT. Insights Imaging 2017;8:557-72.
117. Davidova P, Müller M, Wenner Y, König C, Kenikstul N, Kohnen T. Ophthalmic artery occlusion after glabellar hyaluronic acid filler injection. Am J Ophthalmol Case Rep 2022;26:101407.
118. Danks JJ, Dalgliesh JD, Ayton T. Cosmetic filler blindness: recovery after repeated hyaluronidase injections. Aesthet Surg J 2022;42:411-6.
119. Nguyen HH, Tran HTT, Duong QH, Nguyen MD, Dao HX, Le DT. Significant vision recovery from filler-induced complete blindness with combined intra-arterial injection of hyaluronidase and thrombolytic agents. Aesthetic Plast Surg 2022;46:907-11.
120. Moore RM, Mueller MA, Hu AC, Evans GRD. Asymptomatic stroke after hyaluronic acid filler injection: case report and literature review. Aesthet Surg J 2021;41:NP602-8.
121. Eldweik L. Orbital infarction syndrome following hyaluronic acid filler rhinoplasty. Am J Ophthalmol Case Rep 2021;22:101063.
122. Jolly R, Bhalla M, Zakir R, Joshi N. Visual loss from dermal fillers. Eur J Ophthalmol 2021;31:NP102-5.
123. Zhang L, Luo Z, Li J, et al. Endovascular hyaluronidase application through superselective angiography to rescue blindness caused by hyaluronic acid injection. Aesthet Surg J 2021;41:344-55.
124. Liu H, Chen D, Zhang J. Ophthalmic artery occlusion after forehead autologous fat injection. Retin Cases Brief Rep 2020;14:271-4.
125. Conovaloff J, Hrachova M, Hasso A, Abcede H. Vanishing beauty: a case of a 59-year-old woman with iatrogenic central retinal artery occlusion after cosmetic facial augmentation with filler injections (2694). Neurology 2020:94(15 Supplement).
126. Ramesh S, Fiaschetti D, Goldberg RA. Orbital and ocular ischemic syndrome with blindness after facial filler injection. Ophthalmic Plast Reconstr Surg 2018;34:e108-10.
127. Chen H, Wang H, Yang Z. A case of hyaluronic acid induced blindness with ophthalmoplegia and ptosis. Ophthalmic Plast Reconstr Surg 2018;34:e184-6.
Cite This Article
Export citation file: BibTeX | RIS
OAE Style
Mehta P, Kaplan JB, Zhang-Nunes S. Ischemic complications of dermal fillers. Plast Aesthet Res 2022;9:57. http://dx.doi.org/10.20517/2347-9264.2022.19
AMA Style
Mehta P, Kaplan JB, Zhang-Nunes S. Ischemic complications of dermal fillers. Plastic and Aesthetic Research. 2022; 9: 57. http://dx.doi.org/10.20517/2347-9264.2022.19
Chicago/Turabian Style
Mehta, Preeya, Julie Bass Kaplan, Sandy Zhang-Nunes. 2022. "Ischemic complications of dermal fillers" Plastic and Aesthetic Research. 9: 57. http://dx.doi.org/10.20517/2347-9264.2022.19
ACS Style
Mehta, P.; Kaplan JB.; Zhang-Nunes S. Ischemic complications of dermal fillers. Plast. Aesthet. Res. 2022, 9, 57. http://dx.doi.org/10.20517/2347-9264.2022.19
About This Article
Special Issue
Copyright
Data & Comments
Data
 Cite This Article 55 clicks
Cite This Article 55 clicks



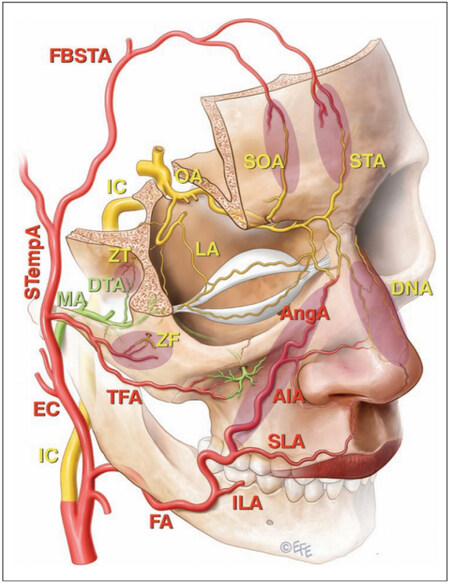
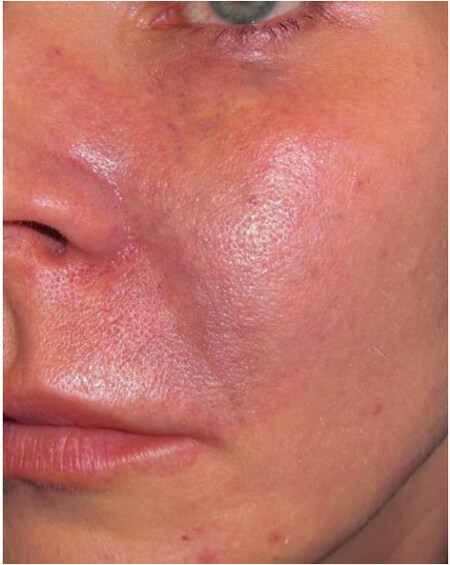
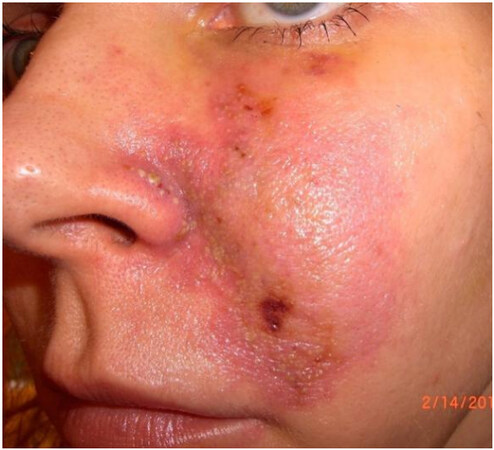
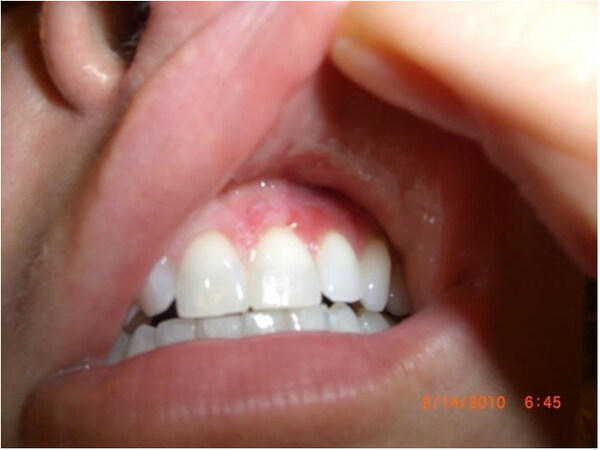
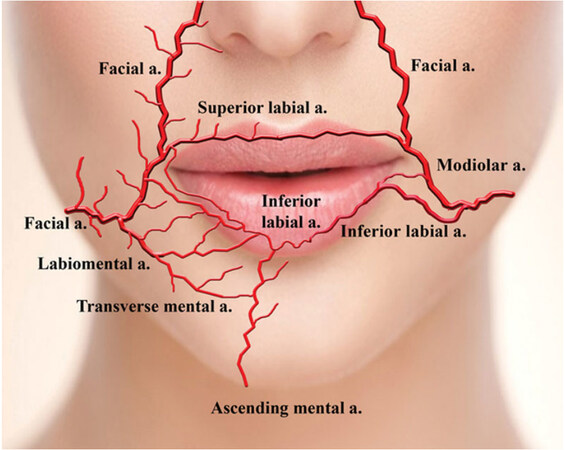
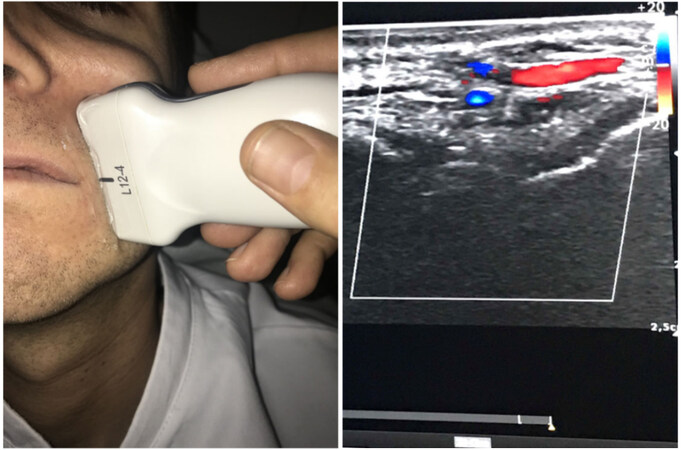
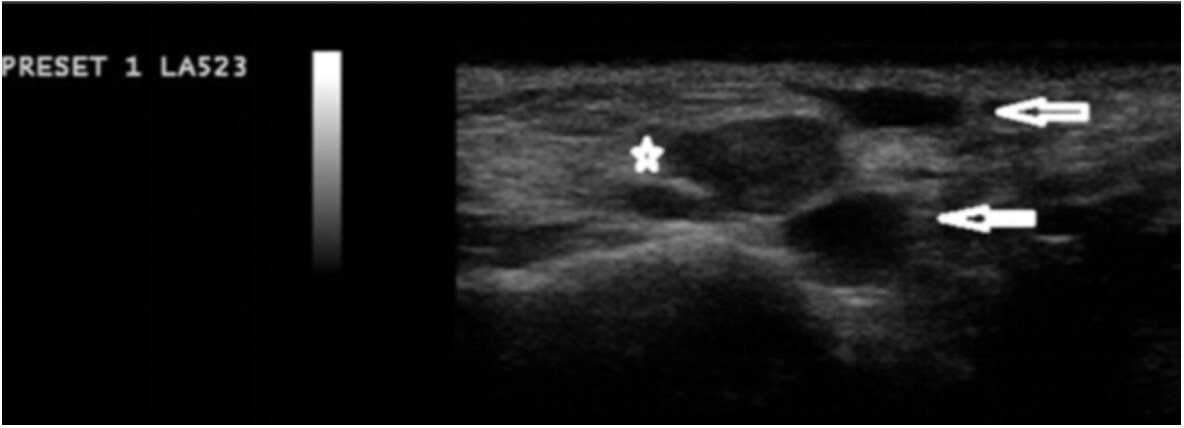
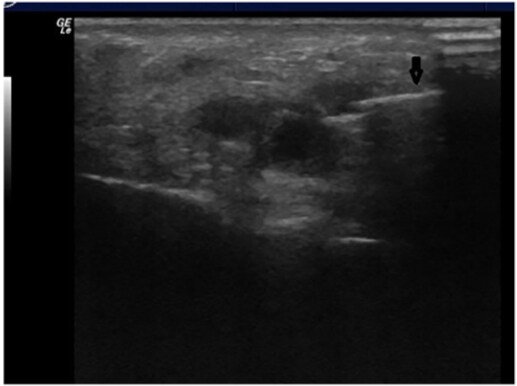
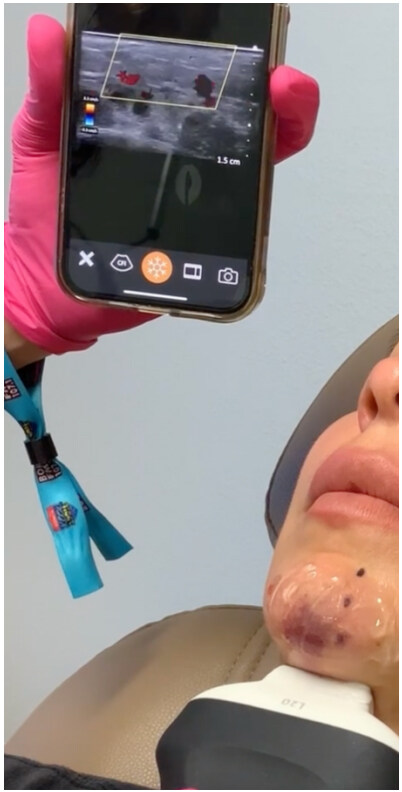









Comments
Comments must be written in English. Spam, offensive content, impersonation, and private information will not be permitted. If any comment is reported and identified as inappropriate content by OAE staff, the comment will be removed without notice. If you have any queries or need any help, please contact us at support@oaepublish.com.