Management of vascular complications following calcium hydroxylapatite filler injections: a systemic review of cases and experimental studies
Abstract
Aim: Dermal fillers are increasingly popular procedures. Inadvertent intraarterial injection of fillers, particularly with calcium hydroxylapatite (CaHA), can result in devastating consequences. A systemic review was performed to summarize management strategies to treat CaHA-associated vascular complications.
Methods: The methodology of this review was derived from The Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA). In addition, this paper presents a previously unreported case of a CaHA-associated vascular complication.
Results: There were 32 articles describing 42 cases, plus our case included in this review. There were 15 cases of vision complications, 23 cases of non-vision complications, and 5 experimental studies. The most common injection sites reported were nasal region for vision complications (45%) and nasolabial folds for non-vision complications (40%). Of the 38 human cases, the most prevalent treatment choice was steroids (24 cases, 63%). Complete or near complete improvement was reported in 83% of non-vision complications and 40% of vision complications. There was no noticeable homogeneity in the management strategies and outcomes of the patients. Of the 5 experimental studies, no clear consensus on treatments was found.
Conclusion: Vascular complications of CaHA are seemingly uncommon, but it is widely suspected that this is due to underreporting. While best management is prevention, preparation for a potential complication is equally important. Derived from CaHA literature, hyaluronic acid filler complication protocols, findings of this review, and personal experiences, this report proposes management strategies for CaHA-associated vascular complications. We hope these strategies provide a much-needed framework for injectors to refer to and utilize as needed.
Keywords
INTRODUCTION
Minimally invasive cosmetic procedures, such as filler injections, have quickly become popular for facial rejuvenation due to their many advantages over cosmetic surgeries. Filler injections are widely available, relatively affordable, have minimal downtime and provide immediate yet subtle improvements in natural appearance[1]. They work by restoring volume in the natural contours of the face or body, thus portraying a more youthful appearance[2].
There are different types of soft tissue fillers categorized into three groups: autologous, biologic, and synthetic[1,2]. The use of synthetic fillers has quickly surpassed the other types due to their long-lasting effects without donor morbidity[2]. Of these, hyaluronic acid (HA) is the most widely used filler, with effects lasting 3-12 months depending on molecular size, cross-linking, and injection location[3]. In contrast, calcium hydroxylapatite (CaHA; Radiesse®, Merz North America, INC., Raleigh, NC) is a filler composed of 30% synthetic CaHA microspheres suspended in a sodium carboxymethyl cellulose carrier gel and lasts an average of 12-18 months[4-6]. This filler is typically long-lasting because fibroblasts appear as the gel is absorbed, and the patient’s own collagen is stimulated to grow, thus rebuilding the structural scaffolding of the subdermal layer of skin[4]. Over time, the CaHA microspheres degrade to their calcium and phosphate subparts and are metabolized by the body’s normal processes[4].
CaHA filler is currently approved by the United States Food and Drug Administration (FDA) for the correction of moderate to severe facial wrinkles and folds, such as nasolabial folds (NLF), for the correction of HIV-associated lipoatrophy, and for hand augmentation to correct volume loss in the dorsum of the hands[4,5,7]. It is also commonly used off-label for wrinkle correction and volume replacement secondary to bone and fat loss in other facial and non-facial areas, such as improving nasal contours, vocal fold augmentation and treatment of stress urinary incontinence[7].
Like all procedures, fillers are susceptible to adverse effects. The most common adverse effects are mild and temporary, such as ecchymosis, edema, erythema, pain, pruritis, contour irregularities, and dissatisfaction[5]. With its increase in popularity, more serious and permanent complications such as vascular trauma and its subsequent effects have become more prevalently documented and dreaded by filler injectors[1,8,9]. Theoretically, HA fillers can be reversed by hyaluronidase, but CaHA fillers do not yet have any equivalent substance for enzymatic degradation[2]. There are three proposed mechanisms of vascular trauma following filler injections: intravascular embolism, extravascular compression, and vascular spasm, of which severe pain, skin blanching followed by dusky, purple discoloration, and cooled skin temperature are common signs and symptoms[8].
There are a few reports summarizing case studies of vascular complications following the injection of CaHA fillers; however, they are either dated or incomplete. This review presents our case report of a patient who suffered a vascular complication following CaHA injection as well as a systemic review of all currently reported vascular complications and their associated management secondary to CaHA injections and all experimental studies proposing a management strategy for CaHA-associated complications.
Case report
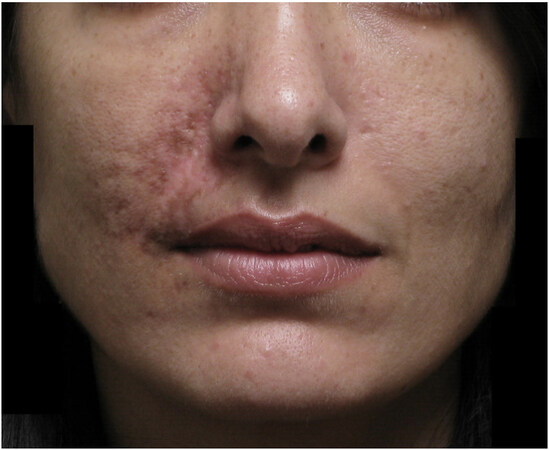
A 28-year-old female received a CaHA filler injection in bilateral NLFs at an unknown medical spa (medi-spa). Within a few days, she reported severe pain and worsening bruising of her right NLF near the injection site. The medi-spa recommend her to an urgent care center for treatment. At the urgent care center, the patient was thought to have herpes simplex or zoster infection and was discharged with oral antibiotics and antivirals. After a few days of worsening skin changes, the patient presented to urgent care again and was transferred to a burn unit for wound care of the developing skin necrosis. Four weeks after the initial injection, the patient sought a second opinion from our office and was diagnosed with a right angular artery occlusion following inadvertent arterial CaHA injection [Figure 1]. Given her late presentation to our office, she was treated with emollient topicals and pulsed dye and non-ablative fractional laser treatments to address the developing scar. A follow-up on the patient six months later showed moderate improvements in skin texture and scarring. Consent was obtained from the patient for the publication of this report.
METHODS
A review of published literature was performed to gather information on the management of vascular complications following inadvertent injection of CaHA filler. The methodology and structuring of this review were derived from The Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) guidelines[10].
Eligibility criteria
Studies were grouped into two categories: case reports with human subjects and scientific intervention studies with animal subjects. Inclusion and exclusion criteria were as follows:
Inclusion criteria:
1. With human subjects - Original articles and posters published up to October 2021, discussing the management of vascular complications following the use of CaHA fillers
2. With animal or cadaveric subjects - Original studies published up to October 2021, evaluating management options for complications following the use of CaHA fillers
3. Articles available in English language
Exclusion criteria:
1. With human subjects - Articles where the discussed complications were not vascular-related
2. Articles where injections of non-CaHA fillers or unknown products were given
3. Articles that did not discuss their management strategy
4. Educational articles with no associated case reports
Information sources and search strategy
The following computerized bibliographic databases: PubMed, Medline, SCOPUS, and EMBASE were searched for reports published up to October 2021 using keywords and Medical Subject Headings (MeSH) search terms relevant to this review. The keywords used in the primary search strategy were: (vascular OR arterial OR venous) AND (occlusion OR insult OR injury OR obstruction OR trauma) AND (“Calcium hydroxylapatite” OR CaHA OR Radiesse OR “Calcium hydroxylapatite filler”). An additional search of the first results page from Google Scholar and Google search engine was executed using the phrases “vascular occlusion ‘calcium hydroxylapatite’”. A complete summary of the full search strategy for all databases and online sources is outlined in [Table 1]. Additional eligible literature not identified in the primary search was searched via a backward, chronological search of the bibliographies of all relevant articles identified in the primary search. All results were limited to those published in English language only.
This table details the full electronic search strategies utilized for all the computerized databases in this review, all conducted on 20 October 2021
| PubMed Advanced Search Builder | ||
| Search string | Results | |
| 1 | Vascular*[tiab] OR arterial*[tiab] OR venous[tiab] AND occlusion*[tiab] OR insult[tiab] OR injury[tiab] OR obstruction[tiab] OR trauma*[tiab] | 1,201,546 |
| 2 | “Calcium hydroxylapatite”[tiab] OR CaHA[tiab] OR radiesse[tiab] OR “Calcium hydroxylapatite filler”[tiab] | 539 |
| 3 | 1 AND 2 | 27 |
| 4 | Limit 3 to (English language) | 27 |
| Medline Advanced Search Builder | ||
| 1 | TX (vascular* OR arterial* OR venous*) AND TX (occlusion* OR insult OR injury OR obstruction OR trauma*) AND TX (“Calcium hydroxylapatite” OR CaHA OR radiesse OR “Calcium hydroxylapatite filler”) | 49 |
| 2 | Limit 1 to (English language) | 49 |
| SCOPUS Advanced Document Search Builder | ||
| 1 | TITLE-ABS-KEY (vascular* OR arterial* OR venous*) AND TITLE-ABS-KEY (occlusion* OR insult OR injury OR obstruction OR trauma*) AND TITLE-ABS-KEY (“Calcium hydroxylapatite” OR caha OR radiesse OR “Calcium hydroxylapatite filler”) | 19 |
| 2 | Limit 1 to (English language) | 19 |
| EMBASE Quick Search Builder | ||
| 1 | (Vascular OR arterial OR venous) AND (occlusion OR insult OR injury OR obstruction OR trauma) AND (“calcium hydroxylapatite” OR caha OR radiesse OR “calcium hydroxylapatite filler”) | 21 |
| 2 | Limit 1 to (English language) | 21 |
| Google Scholar (first page results only) | ||
| 1 | Vascular occlusion “calcium hydroxylapatite filler” | 10 |
| Google Search Engine (first page results only) | ||
| 1 | Vascular occlusion “calcium hydroxylapatite” | 20 |
Study selection, data items, extraction, synthesis, and analysis methods
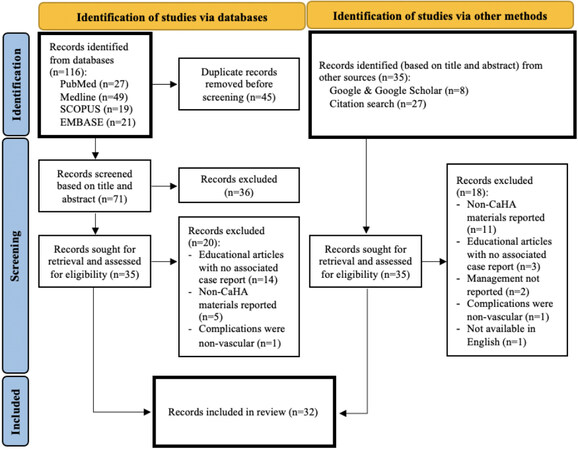
The resulting records from the primary search were first reviewed by one author (Lindgren AL) for the removal of duplicates. Then, titles and abstracts from the search results were screened for relevance, of which 70 full-text documents were obtained. One author (Lindgren AL) read each relevant full-text article and, using the defined eligibility criteria, included 32 articles and excluded 38 articles. Then, Lindgren AL extracted the relevant data from each eligible article and summarized the data in tables. The articles with human subjects were broadly categorized into ocular and non-ocular vascular complications. The data fields for both categories included: author name(s), year of article publication, country of case(s), number of cases, type of filler used, anatomical area of injection, symptoms, and signs of reported vascular complication, management of complication, and case outcome. For articles with animal or cadaveric subjects, the data fields included: author name(s), year of article publication, study objective, substance being tested, study methods, study results, and conclusions. Any missing information was indicated in the tables as “Not Reported (NR)”. Then, two authors (Lindgren AL and Welsh KM) individually evaluated the extracted data to determine final inclusion in this review. Any differences or disagreements were resolved through discussion and consensus between the authors. The selected articles were then qualitatively and quantitatively analyzed by both authors. Derived from PRISMA guidelines, the process of screening, selection, and inclusion of articles and reasons for exclusion were displayed in Figure 2[10]. Due to the small number of cases and evident heterogeneity of the variety of management strategies to address vascular complications following CaHA injection, the findings were presented using tables and narrative summaries.
RESULTS
A total of 42 cases of vascular complications following CaHA injections were extracted from the 32 eligible articles identified in this literature search. Including our patient, this review analyzed 43 total complication cases. The cases were divided into three categories and their characteristics were extracted and summarized in their respective tables. There were 15 cases with vision-related complications [Table 2], 23 cases with non-vision-related complications [Table 3], and 5 cadaveric/animal experimental cases [Table 4].
Summary of data extracted from search results reporting vision-related vascular complication(s) in human subjects following calcium hydroxylapatite injection
| Author(s), year | Country | # of cases | Injection location | Signs & symptoms | Management | Outcome |
| Sung et al., 2010[11] | Korea | 1 | Nasal dorsum | OD: ophthalmoplegia, ptosis, exotropia, chemosis, corneal edema, hyphema, hypopyon, pain & vision loss to hand movements OS: not affected Skin: necrosis and reticulated erythematous pattern on glabella, nasal bridge, and right eyelid | -Aspiration, NS rinses, & wound care -PO steroid -Ophthalmic antibiotic & steroid -IV antibiotic | Skin (minimal scarring) and OD vision improvement to 20/20 with pinhole reported at 3-month follow-up |
| Czyz & Allen, 2011[12] | USA | 1 | Glabella | Pain with injection Pain & edema at injection areas OD: vision loss to 20/30, pain with adduction, conjunctival vessel blanching OS: not affected OD: keratitis, lower lid erythema with ulceration vision improved to 20/20 | -PO anti-coagulant (ASA) -Topical NTG paste & petrolatum -Ophthalmic antibiotic | Skin and OD vision improvement reported at 3-month follow-up |
| Kim & Choi, 2013[13] | Korea | 1 | Nose | OU: vision loss to NLP, ptosis, ophthalmoplegia, blepharoptosis, conjunctival injection Skin: central necrosis with reticulated erythematous pattern on nasal bridge & frontal area | Observation (patient presented late) | No vision improvement reported |
| Chang et al., 2014[14] | Taiwan | 1 | Nose | OU: blurred vision OD: lower visual field defect OS: pain, vision loss to finger counting at 20 cm, Marcus Gunn pupil, full visual field defect, conjunctival injection | -PO anti-coagulant (ASA) & glaucoma agent (acetazolamide) -Ophthalmic antibiotic, steroid, & glaucoma agent (brimonidine) -NS rinses -HBOT (6 sessions) | No OS vision improvement reported at 8-month follow-up |
| Hsiao & Huang, 2014[15] | Taiwan | 1 | Glabella | OD: not affected OS: vision loss to hand motion at 15 cm, dilated pupil, RAPD | -PO anti-coagulant (ASA), glaucoma agent (unspecified), & steroid -Ophthalmic glaucoma agent -Isovolemic hemodilution -Ocular massage -Carbogen inhalation therapy -HBOT (6 sessions) | OS vision improvement to 20/200 at 3-month follow-up |
| Chou et al., 2015[16] | Taiwan | 1 | Nasal tip & nasal dorsum | Nausea, vomiting, headache OD: not affected OS: pain, vision loss to hand motion at 30 cm, exotropia, ophthalmoplegia, sluggish pupillary light. reflex, RAPD, Skin: necrosis over nasal dorsum, glabella, & left forehead | -IV vasodilator (alprostadil) & anticoagulant (dextran) -HBOT (10 sessions) | OS vision improvement to 20/200 at 1-month follow-up |
| Hsieh et al., 2015[17] | Taiwan | 2 | Glabella | Headache, general weakness OD: not affected OS: vision loss to NLP, ophthalmoplegia, ptosis, pain OS: hypotony Skin: multiple reticulated, erythematous-to-violaceous ulcerative skin lesions over glabella, perinasal, & periorbital areas | -PO anti-coagulant (ASA) & glaucoma agent (acetazolamide) -Ophthalmic glaucoma agent (timolol) -Hydration -Ophthalmic steroid | No OS vision improvement reported at 3-week follow-up |
| Nasal bridge | OD: not affected OS: vision loss to 20/60, pain, lower visual field defect OS: vision loss to 20/50, hypotony | -PO anti-coagulant, glaucoma agent (unspecified), & steroid -Ophthalmic glaucoma agent & steroid -HBOT | Worsened OS vision to 20/200 at 2-month follow-up | |||
| Cohen et al., 2016[18] | Israel | 1 | Nasal bridge | OD: pain, blurred vision OS: not affected OD: vision 20/20, ptosis, exotropia, ophthalmoplegia Skin: bruising on nasal bridge & forehead, periorbital hematoma | -Aspiration, warm compresses, ocular massage -PO anti-coagulants (ASA, enoxaparin), antibiotic, steroid -Ophthalmic antibiotic -Topical antibiotic | Worsened OD vision to 20/60 at 18-month follow-up |
| Glass et al., 2017[19] | USA | 1 | Temples, cheeks, forehead, chin | Nausea, vomiting, pain Skin: hematoma at right temple OD: vision loss to 20/25, ptosis, diplopia, ophthalmoplegia OS: not affected | -PO steroid | Improvement of skin and ocular symptoms reported at 2-month follow-up |
| Marumo et al., 2018[20] | Japan | 1 | Glabella | Nausea, impaired consciousness OD: not affected OS: vision loss to 20/200, diplopia, ophthalmoplegia, conjunctival injection, dilated pupil Skin: purpura from glabella to left forehead OS: vision loss to hand motion, hypopyon, hyphema Skin: necrosis from glabella to left forehead | -PO steroid -Ophthalmic steroid -Topical ointment & wound care | Skin and OS vision improvement to 20/25 at 2-month follow-up |
| Sunget al., 2018[21] | Taiwan | 1 | Nasal bridge | Headache, vomiting OD: not affected OS: vision loss to 20/63, diplopia, pain, ophthalmoplegia, exotropia Skin: bruising along nose to glabella & forehead | -PO steroid & antibiotic -HBOT | Skin and OS vision improvement to 20/20 at 2-month follow-up |
| Vu et al., 2018[22] | USA | 1 | Glabella & nasal dorsum | Nausea, vomiting, headache OD: vision loss to NLP, conjunctival injection & chemosis, afferent pupillary defect OS: not affected Skin: discoloration of right forehead, glabella, & nasal dorsum | -Hyaluronidase injections, ocular massage -PO anti-coagulant (ASA) & steroid -Topical NTG paste -HBOT | Skin and OD vision improvement to light perception at 3-month follow-up |
| Oh et al., 2019[23] | USA | 1 | Glabella & eyelid areas | OD: vision loss to NLP, RAPD, nonreactive pupil OS: not affected | Observation (patient presented late) | No OD vision improvement reported at 9-month follow-up |
| Liu et al., 2020[2] | Taiwan | 1 | Nasal dorsum | Nausea, vomiting OD: vision loss to 20/50 OS: vision loss to 20/63, ophthalmoplegia, severe pain Skin: ecchymosis over nasal dorsum | -IV steroid, vasodilator (alprostadil), & antibiotic -Ophthalmic antibiotic & glaucoma agent (timolol) -Topical antibiotic -HBOT | OU vision improvement to 20/20 at 4-day follow-up Skin & ophthalmoplegia improvement reported at 2-month follow-up |
Summary of data extracted from search results reporting non-vision-related vascular complication(s) in human subjects following calcium hydroxylapatite injection
| Author(s), year | Country | # of cases | Injection location | Signs & symptoms | Management | Outcome |
| Georgescu et al., 2009[24] | USA | 2 | Glabella | Bruising & pain in injection area Skin necrosis along right supratrochlear artery | -PO steroids -Topical NTG paste -Microdermabrasion | Some improvement (residual hyperemia) reported at 4-month follow-up |
| NLF | Pain, edema, ecchymosis, & necrosis along right angular artery | -PO antibiotic & steroid -Topical steroid -Microdermabrasion | Full improvement reported at 4-month follow-up | |||
| Winslow, 2009[25] | USA | 1 | Nose | Blanching | -Topical NTG paste | Full improvement reported at 2-week follow-up |
| Dayan et al., 2011[26] | USA | 3 | NLF & infraorbital region | Blanching over left cheek, left NLF, & left upper lip Upper lip tenderness, edema & erythema of left lower face; reticulated vascular congestion of upper lip & left buccal mucosa | -Hyaluronidase injection -PO anti-coagulant (ASA) & steroid -Topical NTG paste & oxygen infusion cream | Full improvement reported at 5-day follow-up |
| NLF | Soreness, edema, erythema, thick serous drainage of right NLF, erythematous & congested reticular pattern of overlying skin | -Betadine, warm compresses -Hyaluronidase injections -PO anti-coagulant (ASA) & antibiotic -Topical NTG paste, oxygen infusion cream & antibiotic | Full improvement reported at 4-week follow-up | |||
| NLF | Edema, erythema, & bruising of right NLF Worsening edema & erythema, skin breakdown & ulceration Large patch of reticulated skin, mild edema, atrophic skin | -IV antibiotic -PO antibiotic & antiviral -Topical steroid -Hyaluronidase injection -PO anti-coagulant (ASA) & antibiotic -Topical NTG paste & oxygen infusion cream | Full improvement reported at 8-week follow-up | |||
| Beer et al., 2012[27] | 2 | NLF | Blanching & pain along right angular artery Superficial erosions & small yellowish papules | -PO anti-coagulant (ASA), steroid, antibiotic, & antiviral -Pulsed dye & fractionated erbium laser sessions | Full improvement reported at 4-month follow-up | |
| NLF | Blanching along right angular artery | -Hyaluronidase injections, massage -Incision and drainage -Topical NTG paste -PO anti-coagulant (ASA), vasodilator (sildenafil) & steroid -Topical antibiotic -HBOT (10 sessions) | Some improvement (residual bruising) reported at 2-week follow-up | |||
| Darling et al., 2014[28] | USA | 1 | Cheek | Eroded plaque on right cheek | -PO anti-coagulant (ASA), vasodilator (pentoxifylline) antibiotic, & antiviral -Topical NTG paste & petrolatum -Red light therapy -HBOT (14 sessions) -Pulsed dye & fractionated erbium-doped laser sessions | Full improvement reported at 11-week follow-up |
| Tracy et al., 2014[29] | USA | 1 | NLF | Swelling & skin changes of left NLF Frank tissue necrosis, diffuse inflammation, & fibrinous exudate of left NLF | -PO steroid, antibiotic & antiviral -Wound care & debridement -Pulsed dye laser sessions | Full improvement reported at 4-month follow-up |
| Schuster, 2015[30] | Germany | 2 | Nasal radix & columella | Painless red nasal tip | -PO antibiotic -Topical steroid | Full improvement reported at 4-week follow-up |
| Nasal dorsum | Local infection at injection area & punctate skin lesions Worsening infection | -Topical disinfection & antibiotic | NR (Lost to follow-up after 3rd day) | |||
| Dominguez et al., 2017[31] | USA | 1 | NLF | 30 seconds of blurred vision (then, OU vision 20/20), epiphora, blanching of left cheek & upper lip, fine touch & pinprick sensation of left cheek diminished | -PO anti-coagulant (ASA), vasodilator (sildenafil), & steroid -Subcutaneous anti-coagulant (enoxaparin) -Topical NTG paste -HBOT | Full improvement reported at follow-up (unknown date) |
| Rocha & Hirano, 2019[32] | Brazil | 1 | Temples | Intense pain & vivid red telangiectatic discoloration Ecchymosis | -Hyaluronidase injections, compresses -PO anti-coagulant (ASA), vasodilator (sildenafil) & steroid | Full improvement reported at follow-up (unknown date) |
| Uittenbogaard et al., 2019[33] | Nether-lands | 1 | Unreported facial location | Burning pain, numbness, & white discoloration in injected area | -Hyaluronidase injections, warm compresses -PO vasodilators (sildenafil, nifedipine) & steroid -HBOT (10 sessions) | Full improvement reported at 6-month follow-up |
| Schelke et al., 2020[34] | Nether-lands | 4 | Cheek | Skin changes (reticulated blueish pattern with/without pustules & wounds) & doppler-ultrasound images (hypervascular turbulent artery with/without detectable filler blockage) along transverse facial artery | -STS injections (250 mg/mL- 0.2 mL/cm2) · 2 | NR |
| Cheek | Skin changes (reticulated blueish pattern with/without pustules & wounds) & doppler-ultrasound images (hypervascular turbulent artery with/without detectable filler blockage) along transverse facial artery | -STS injections (250 mg/mL- 0.2 mL/cm2) · 2 | NR | |||
| Tongue | Skin changes (reticulated blueish pattern with/without pustules & wounds) & doppler-ultrasound images (hypervascular turbulent artery with/without detectable filler blockage) along submental artery | -STS injections (250 mg/mL- 0.2 mL/cm2) · 2 | NR | |||
| Chin | Skin changes (reticulated blueish pattern with/without pustules & wounds) & doppler-ultrasound images (hypervascular turbulent artery with/without detectable filler blockage) along infralabial artery | -STS injection (250 mg/mL- 0.2 mL/cm2) · 1 | NR | |||
| van Loghem et al., 2020[35] | USA | 2 | NLF | Erythema & reticulated skin changes of left nasal ala and NLF Vascular necrosis & ischemia | -Massage & warm compresses -PO vasodilator (sildenafil), antibiotic & antiviral -Topical NTG paste & petrolatum | Full improvement reported at 2-month follow-up |
| Nasal bridge & columella | Slight blanching of upper lip Reticulate livedo & cyanosis Edema, blue discoloration & decreased sensation Increased edema & burning sensation of upper lip, nose tip numbness | -Hyaluronidase injections -PO vasodilator (sildenafil) -IV vasodilator (pentoxifylline) -Intramuscular steroid -Topical wound jelly (Solcoseryl®) -HBOT (1 session) | Full improvement reported at 2-week follow-up | |||
| Williams & Burgess, 2021[36] | USA | 1 | Cheek & infraorbital region | Bruising & dull aching pain in right cheek Pain, crusted erythematous plaque on right cheek, reticulated non-blanching erythema of right infraorbital region; injected right sclera (OU vision normal) | -NS rinses -Topical NTG paste -Red light therapy (8 sessions) | Full improvement reported at 6-month follow-up |
| Our Case | USA | 1 | NLF | Severe pain, bruising Textural skin changes and scarring | -PO antibiotic & antiviral -Wound care -Pulsed dye and non-ablative fractional laser | Some improvement reported at 6-month follow-up |
Summary of data extracted from search results reporting treatment experiment(s) for vascular complications following calcium hydroxylapatite usage in human cadaver and/or animal subjects included in this review
| Author(s), year | Objective | Methods | Results & conclusions |
| Hwang et al., 2016[37] | To evaluate vascular perfusion effects of NTG ointment following filler-associated vascular occlusion & ischemia in rabbit ears | All rabbit ear models were injected with four different soft-tissue fillers to create skin ischemia. After 20 min, models received one of the following: -Application of topical NTG ointment 2% -No treatment to serve as controls Vascular perfusion images using ICG imaging were obtained at baseline, immediately after, & 30 min after filler injection, & again at 30, 60, 90, & 120 min after application of NTG ointment | No statistically significant improvement in perfusion was noted after topical application of NTG with ICG imaging Topical NTG could possibly worsen ischemia as vasodilation could propagate product into smaller vessels. NTG paste also has systemic effects, which some patients may not tolerate |
| Kreymereman & Miller, 2018[38] | To study efficacy & concentration of intralesional STS for dissolving CaHA particles in vivo & to assess any histologic changes | Animal models received intralesional injections of CaHA (0.5 mL/site). Models then received one of the following: -0.25 mL of 25% STS solution injection at 1 h, 24 h, or 7 days after CaHA -0.50 mL of 25% STS solution injection at 1 h, 24 h, or 7 days after CaHA -No STS injection to serve as controls Samples were collected via 5-mm punch biopsy, then stained & examined by light microscopy. A semiquantitative 5-point scale was used to estimate the number of CaHA microspheres present | CaHA particles had the greatest reduction with the 0.5 mL STS doses 1 h after CaHA injection. Reduction was greater with the 0.5 mL STS dose compared to the 0.25 mL dose. Reduction of CaHA particles was less pronounced at 24 h & 7 days after CaHA injection No CaHA-related histologic changes were observed |
| Robinson, 2018[39] | To determine whether intralesional STS & topical SMB may help dissolve CaHA in porcine skin models | Cadaveric porcine skin models were injected with CaHA (0.4-0.8 mL). Models then received one of the following: -0.2 mL of intralesional STS (12.5 g/50 mL) -1-2 g of topical SMB (25% SMB in 120 mL gel) applied with occlusion -Both intralesional STS & topical SMB -No treatment to serve as controls Samples were collected via 4-mm punch biopsy 24 h after treatment & then stained & examined by light microscopy. A board-certified dermatopathologist estimated the amount of CaHA present in each sample | Intralesional STS alone or combined with topical SMB completely dissolved CaHA in porcine skin models. Topical SMB treatment partially reduced CaHA from tissue samples Intralesional STS alone was as effective as STS & SMB combination, indicating that intralesional STS could possibly be used to dissolve CaHA dermal filler |
| Danysz et al., 2020[40] | To verify previous findings suggesting the efficacy of intralesional STS in the reduction of CaHA volume & nodule formation | In vitro → Three systems (glass tubes, human skin equivalent, & human ex vivo skin) were each administered CaHA microspheres. Models in each system were then administered one of the following: -Aqua distilled water -Phosphate-buffered saline (PBS) -25% STS -0.1 M ethylenediamine tetraacetic acid (EDTA) solution to serve as controls In vivo → Live pig models were injected with 500 μL of CaHA. An hour later, models received one of the following: -1500 μL of STS -1500 μL of saline -No treatment to serve as controls In vitro samples were collected after 3 days & in vivo samples after 7 days. Samples were analyzed via 3D camera, histopathology, scanning electron microscopy, & computer tomography in vivo (CT) & micro-CT ex vivo | Results suggest no indication of CaHA degradation by STS, either in vitro or in vivo Histology, micro-CT ex vivo, & CT in vivo indicated a decrease in CaHA volumes after STS treatment, which authors attributed to dispersion effect Observed necrosis & hemorrhaging are further obstacles to the use of STS |
| Yankova et al., 2021[41] | To test whether CaHA fillers can be trans-arterially dissolved by STS when evaluated in cadaveric & in vitro models | Cadaveric → Human cadaveric facial arteries were filled with 0.2 cc of CaHA & submerged in the following solutions: -10 cc STS (300 mg/cc) (pure) -5 cc STS (300 mg/cc) & 5 cc 0.9% saline (1:1 dilution) -3.3 cc STS (300 mg/cc) & 6.6 cc 0.9% saline (1:2 dilution) -5 cc STS (300 mg/cc) & 5 cc 300 IU (bovine) hyaluronidase (1:1 ratio) In vitro → 0.5cc CaHA injected directly into the four solutions listed above Samples were collected after 24 h. The cadaveric arteries were dissected and visually analyzed for CaHA product. In vitro samples were analyzed via gray-scale imaging | CaHA was detected after 24 h, independent of the STS concentration. STS & hyaluronidase combined also did not dissolve the CaHA product. Gray-scale analyses from the in vitro testing showed that higher STS concentrations resulted in increased disintegration of CaHA Results indicate STS has limited potential to dissolve intraarterial CaHA of cadaveric facial arteries, even though it appears effective when in direct contact with CaHA |
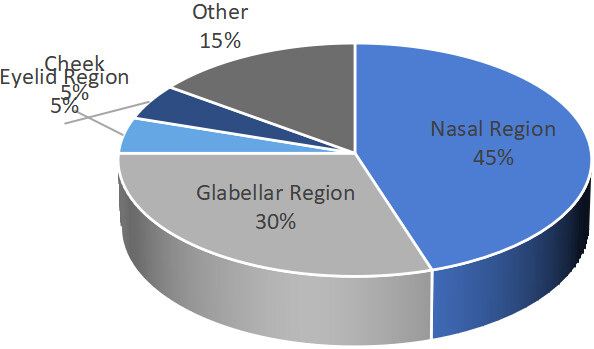
Of the 15 cases with vision complications, there were 20 injection locations reported, with the two most common sites being the nasal region (9 cases, 45%) and glabellar region (6 cases, 30%). The summary of all sites is depicted in Figure 3. Administration of steroid(s) was the most common management strategy reported in 12 of these cases (80%), followed by anti-coagulating agent(s) (8 cases, 53%), hyperbaric oxygen therapy (HBOT; 7 cases, 47%), and antibiotic medication(s) (6 cases, 40%). Other reported treatments included anti-glaucoma agents, oral vasodilating agents, topical nitroglycerin (NTG), ocular massage, aspiration, and various wound care strategies. Of note, two of the 15 cases reported observation as the only management and these cases had no improvement in their respective complications[13,23].
Figure 3. Reported injection locations of calcium hydroxylapatite (percentage; %) extracted from cases of vision-related vascular complications.
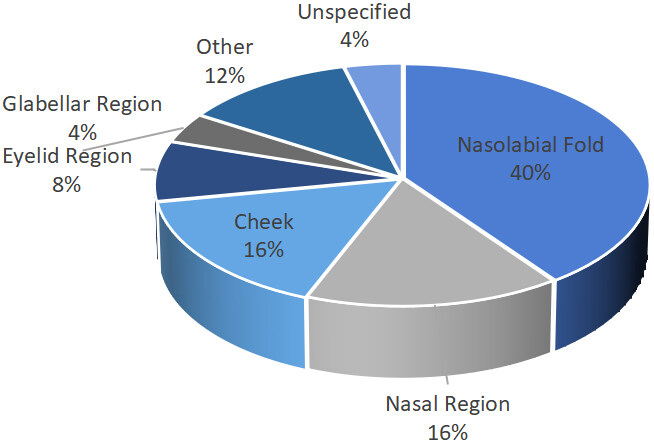
Of the 23 cases with non-vision-related complications, the 25 reported injection locations were depicted in Figure 4, with the most common sites being NLF (10 cases, 40%), nasal region (4 cases, 16%), and cheek
Figure 4. Reported injection locations of calcium hydroxylapatite (percentage; %) extracted from cases of non-vision-related vascular complications.
Addressing all 38 of the case reports, there was no noticeable homogeneity in the timeline of management strategies with care ranging from minutes to months after injection administration depending on patient presentation. Outcomes were reported in 33 of the 38 cases (87%), of which the final follow-up times ranged from 5 days to 18 months with an estimated average of 3.5 months. It is also interesting to note that 15 of the 18 non-vision-associated cases that reported outcomes (83%) described complete improvement of signs and symptoms at follow-up, while only 6 of the 15 vision-related cases (40%) reported complete or near-complete resolution of visual defects.
This review also identified 5 experimental studies with cadaveric or animal subjects that investigated the efficacy of treatments in dissolving CaHA. One study tested topical NTG ointment, and the other four studies tested sodium thiosulfate (STS). The STS study by Robinson[39] additionally investigated the effects of topical sodium metabisulfite (SMB) in combination and in isolation from intralesional STS. Topical SMB was not found to be helpful in CaHA-associated complications. Results were varied in the four STS studies. One found complete dissolution of CaHA with STS[39], one found partial reduction[38], and the remaining two found no evidence indicating STS could dissolve CaHA[40,41]. Of note, the two studies that found no evidence supporting STS were the most recently published in 2020 and 2021, respectively[40,41].
DISCUSSION
Complication prevalence
Inadvertent intraarterial injection of filler material is an uncommon but severe complication. Vascular complications specifically following CaHA injections seem to be even more rare, with only 43 cases identified in our comprehensive search compared to hundreds of reported vascular complication cases following other filler types[1,42]. The true prevalence of CaHA-associated complications is unknown, in part, due to the widely accepted theory that complications are underreported in the literature[42,43]. Antidotally, this is thought to be due to a reluctance to report stemming from embarrassment or evidence of poor technique[42]. Unlike the reversal action of hyaluronidase on HA-fillers, CaHA fillers do not have an antidote and severe complications are possibly more likely to occur following inadvertent injections. Therefore, another possible theory is that CaHA complications often require a greater immediate focus on the patient’s wellbeing and reporting seems inconsequential at the time. Regardless of the reason, it is important to report complications so wary injectors can stay informed.
Mechanism of CaHA-associated vascular complications
Current consensus suggests that inadvertent injection of CaHA into arterial vessels can result in filler emboli formation and occlusion via retrograde flow against the blood pressure to a point or past the point of vessel branching[1,44]. Subsequent release of syringe pressure allows the patient’s blood pressure to reestablish antegrade flow, which potentially can further push the emboli into smaller peripheral vessels[1]. The final resting place of the emboli determines the location and extent of ischemia that occurs. Many factors influencing the etiology and outcome of vascular complications have been suggested. The location selection of the injection is thought to be highly influential in complication outcomes as some facial areas are more vascular and considered at higher risk than other areas[1]. The size of the needles, syringes, and cannulas used for injection has been suggested to influence the risk of vascular trauma[44]. Finally, the volume, speed, and pressure of the injection are all thought to impact the retrograde flow of the filler and, therefore, the potential extent of vascular compromise[44].
Preventative management
The best management is, of course, prevention. Injectors should have a thorough knowledge of facial and non-facial vascular anatomy, its common variants, and the safest tissue planes for injection. It is highly suggested that injectors regularly attend training courses to learn and practice the safest and most up-to-date injection techniques, in addition to monitoring literature for updates on techniques and management. One example is the growing appreciation for handheld Doppler Ultrasound analysis devices to map the facial vasculature prior to filler injections[45,46]. It is important to note that while the use of technology like this has the potential to supplement anatomical knowledge and training, it should never replace this education.
As evidenced by the cases in this review, all areas of the face have the potential for inadvertent intraarterial injection, but injectors should be particularly wary of high-risk areas when selecting filler placement. Among all 45 reported injection sites reported in the 38 cases, complications were mostly attributed to the nasal region (15 sites, 33%), NLF (10 sites, 22%) and glabellar region (7 sites, 16%). The nasal and glabellar regions are known high-risk areas, but it was surprising to see nasolabial fold in the top three as it is generally considered a safe injection location. Given the FDA approval for CaHA in NLFs, it is likely that there has been a much larger absolute number of injections to NLF compared to other facial areas, and the proportion of NLF-associated complications seems more significant than it really is in this study. Of note, 8 of the 10 NLF-associated cases reported full resolution, one case reported residual bruising at a 2-week follow-up, and one case resulted in visible scarring. CaHA injection in the nasal and glabellar regions is considered high-risk due to the complex vascularity in these facial areas. The angular artery of the nasolabial fold area branches into the dorsal and lateral nasal arteries which supply the nasal region, and the supratrochlear artery which supplies the glabella. These branches connect to the ophthalmic artery which in turn branches into the central retinal artery, a terminal vessel critical in supplying the retina. This high-risk label for these areas was supported by this review. Fourteen out of the 15 case reports (93%) affecting vision involved the glabella, nasal region, or both. Of those 14 cases, 5 patients (36%) had resolution of vision to 20/25 or better, one patient had worsened vision to 20/60, and 8 patients (57%) were reported to be legally blind or worse.
Best injection practices continue before the needle even pricks the skin. The utility of blunt, flexible cannulas versus sharp needles is thought to minimize intraarterial perforation[47]. Additionally, the size selection of the cannula influences outcomes. Studies found that the force needed to penetrate vessels was greater for most cannulas compared with correspondingly sized needles and significantly decreased with smaller diameter cannulas compared to large diameters[48,49]. In practice, this means that cannulas are considered safer than needles until they are about 27-gauge and smaller, at which point, the cannula is close to the sharpness of a needle[48,49]. It is also thought that the application of a local vasoconstrictor and/or tumescent injection with saline before filler injection can help reduce the risk of arterial puncture and vascular trauma[44,50].
When the needle is finally ready to pierce the skin, it is a common practice and is even encouraged to aspirate before the filler is injected into the skin. While this is widely accepted, it is important to be wary that aspiration is extremely variable in its sensitivity, depending on the gauge and length of tools used, thickness of the filler, and length of time allowed for observation of the aspirate. Therefore, a negative aspirate is no guarantee of safety[51,52]. Injection placement, particularly with cannulas, should not parallel arteries and should not be attempted if met with increased resistance or patient pain. Special caution should also be taken in pre-traumatized areas of skin from past surgery or injury where landmarks and vascular anatomy may be disturbed[44].
Once filler injection begins, it is advised to inject slowly and in small increments[8,42,44]. Smaller injection volumes of filler are thought to be less obstructive and allow blood to bypass via collateral vessels[42]. Injections should also be slow and with minimal pressure exerted to avoid retrograde propulsion of filler emboli into an artery[42,44]. Injectors should actively observe for signs of arterial occlusion, including blanching, mottling, severe bruising, extraordinary pain, evidence of peripheral reperfusion, and/or fluctuations in vision[8,44]. Delayed vision changes and/or pain with or without severe bruising can also be signs of delayed embolization and associated vascular occlusion[8].
Review of post-complication management therapies
Anti-inflammatory agents
Steroids
In the case of vision loss and retinal edema, 1 g intravenous methylprednisolone is a refractory treatment option reported in the literature, but it is usually reserved for inflammatory artery occlusions and there is no evidence of its positive effect on ischemic outcomes[53]. Notably, 11 of the 15 vision-related case reports (73%) administered steroids with outcomes ranging from full vision restoration to worsened vision. In cases of retinal ischemia, steroid administration is not recommended in ocular cases without the input of an ophthalmologist.
Sodium thiosulfate
Anti-coagulant/Thrombolytic agents
Acetylsalicylic acid (aspirin)
Low molecular weight heparin
Treatments that increase blood oxygen
Hyperbaric oxygen therapy
Phosphodiesterase type 5 inhibitors
Prostaglandin E1 analogs
Calcium channel blockers
Nitroglycerin
Of note, none of the case reports in this review utilized sublingual NTG in their management plans despite its potential effectiveness seen in central retinal artery occlusion from other thromboembolic events[59]. Given the poor vision prognosis of CaHA-induced central retinal artery occlusion, administering sublingual NTG should be considered when there are no contraindications.
Pentoxifylline
Carbogen inhalation
Tissue massage and/or warm compress
Treatments that reduce intraocular pressure
Ophthalmic glaucoma drops
Acetazolamide
Ocular massage
Anti-microbial agents
Antibiotics
Antivirals
Wound and scar care
Open wound care
Interestingly, eight cases (21%) administered hyaluronidase flushes despite no evidence that hyaluronidase can break down or dissolve CaHA fillers. It is thought that hyaluronidase has been used in non-hyaluronic filler incidents for its edema-reducing and anti-inflammatory properties[35]. For ischemic events involving skin tissue, a dose of 600 units of hyaluronidase per 0.1 mL CaHA is recommended right after the event and every two hours up to four cycles if no improvement is seen[35]. For ocular ischemic events, there is no known evidence or consensus that hyaluronidase would aid in ischemia reversal; therefore, its usage is not recommended without the consultation of an ophthalmologist.
Closed wound care
Despite proper wound care, the healing process of skin often results in scar formation which can be a traumatic burden to the patient[35]. At this stage, treatments such as microdermabrasion, pulsed dye laser, non-ablative fractioned laser resurfacing, and fractionated laser resurfacing can be used monthly to improve texture and scarring[35]. Seven of the ischemic skin complication cases reported using at least one type of microdermabrasion or laser treatment, of which five cases reported complete resolution. The timing of follow-up and the extent of skin damage may explain these varied outcomes. Although not used in the case reports of this review, topical treatments such as growth factors and vitamin C should also be considered to supplement laser treatments[35]. Early and consistent intervention is recommended for best results. It is also important to advise the patient that underlying collagen production is a slow process and satisfactory results may take 10-12 months to achieve[35].
Summary of management recommendations
There is not a gold standard for treating vascular complications following CaHA injections. While prevention is the best strategy, it is also important to have a management plan prepared to quickly respond to these potential complications. The timing of management could mean the difference between recovery and a permanent deficit. As a result, this review presents the following management recommendations derived from the findings of this review, current literature[31,35,44,61-63] proposed protocols for HA-associated intraarterial occlusions[8], and the authors’ personal experiences.
Skin tissue ischemia: discoloration, mottling, extraordinary pain, etc.
1. If acute, stop the injection and attempt aspiration before withdrawing the needle
2. Assess capillary refill time by gently compressing the skin of the affected area for 5 s
a. normal refill time is 3-4 seconds or less
b. If refill time is greater than 4 seconds, proceed to the next steps
3. Administer the following as soon as possible:
a. Apply warm compresses and gently massage the area for 5 minutes every hour
b. Flood the area with hyaluronidase 600 units per 1cc of CaHA injected
i. If reperfusion is not noted, repeat every 2 h up to 4 times
c. Administer the following as soon as possible:
i. Oral aspirin 500-650 mg plus antacid (If contraindicated, consider subcutaneous LMWH)
ii. Oral prednisone 50-60 mg
iii. Oral pentoxifylline 40 mg
iv. Oral PDE5 inhibitor 20 mg
4. If circulation is restored, follow-up weekly up to 4 weeks
a. If vascular compromise persists or is delayed, proceed to the next steps
5. Continue treatments:
a. Oral aspirin 75 mg daily for 3-5 days
b. Oral prednisone taper over 7 days
c. Oral PDE5 inhibitor 20 mg for 3-5 days
d. Wound care and debridement as needed
6. Consider supplementing with the following:
a. HBOT 1-2 sessions per day up to 10 days
b. Topical and systemic antibiotics
c. Antivirals (especially if the patient has a history of Herpes simplex)
d. Red LED light as soon as 2 days after event
e. Bipolar radiofrequency, microdermabrasion, and/or laser resurfacing treatments for scarring as needed
Retinal ischemia: vision loss, ocular pain, etc.
1. If acute, stop the injection and attempt aspiration before withdrawing the needle
2. Immediately prepare transport to a hospital or known ophthalmologist - there is an approximate window of 60-90 min for recirculation before permanent vision loss
a. Document visual acuity for each eye, injection time, product(s) used, injection site(s), volume administered, and any in-office medications administered
3. Until transfer:
a. Apply firm pressure to the affected eye(s) for 10-15 s, then release for 3-5 s, repeat until transfer
b. Carbogen rebreathing with paper bag
c. Administer oral aspirin 650 mg plus antacid (If contraindicated, consider subcutaneous LMWH)
d. Administer 2 drops to the affected eye(s) of either ophthalmic Timolol 0.5% OR Brimonidine 1%
e. Consider sublingual NTG
4. In hospital:
a. Intravenous (IV) dexamethasone 1 g
b. IV acetazolamide 500 mg
c. Consider:
i. Central nervous system (CNS) imaging
ii. Systemic anti-coagulant
iii. IV prostaglandin E1 analog
iv. Within the first 8 h, a 90-min session of HBOT at 3 atm
v. Retrobulbar flush of hyaluronidase 2-4 mL (150-200 units/mL)
vi. Anterior chamber paracentesis
vii. Neodymium: yttrium-aluminum-garnet (Nd-YAG) laser
5. Discharge:
a. 1-2 drops to the affected eye(s) 4 times a day for 7 days of ophthalmic Timolol 0.5% OR Brimonidine 1%
b. Consider:
i. Oral aspirin 75 mg daily for 3-5 days
ii. HBOT 1-2 sessions per day up to 10 days
iii. Ophthalmic antibiotic drops
6. In addition to ophthalmology, consider consulting neurology and plastic surgery
7. Follow-up with the patient for dermatological care as needed
Limitations
There were many limitations to this systemic review. There was no homogeneity in the presentation, management, or follow-up time of the case reports and the experimental studies. In the cases with visual complications, a few did not report the initial visual acuity of their patient, and fewer authors reported data that indicated a basic eye examination was completed. Thus, this review could not infer a cause-and-effect relationship, a common limitation with descriptive studies. Lastly, these findings cannot be generalized as the information generated from these case reports is not representative of the entire population.
In conclusion, vascular complications following CaHA injections are seemingly rare but often associated with devastating outcomes. There is no gold standard for treating CaHA-associated complications, and even though this review outlines recommendations, there is no known universally effective management plan for these complications. Preventative measures remain the best practice. When vascular occlusions do occur, early recognition and prompt treatment are paramount to minimizing consequences. If there are any ocular symptoms following CaHA injections, immediate referral to an ophthalmologist is highly recommended.
DECLARATIONS
AcknowledgementWe would like to acknowledge Dr. Sidharth Puri for his ophthalmological insight.
Authors’ contributionsMade substantial contributions to the concept of this article; the acquisition, analysis, and interpretation of data for this article; and the drafting of this article: Lindgren AL, Welsh KM
Availability of data and materialsThe data that support the findings of this study were derived from resources detailed in the text. The details of these resources are available in the “References” section of this text and can be sourced from PubMed, Medline, SCOPUS, EMBASE, Google Scholar and/or Google search engine. Additionally, data are available from the corresponding author, Welsh KM, upon reasonable request.
Financial support and sponsorshipNone.
Conflicts of interestBoth authors declared that there are no conflicts of interest.
Ethical approval and consent to participateThe authors made every effort to ensure the best possible quality, integrity, and impartiality of the information in this review. The authors discussed and mitigated risks and potential harm to the human participant referred to in the case report. The authors respect the confidentiality and anonymity of the included participant, ensured participation was voluntary, and obtained informed consent to be included in this text from the participant.
Consent for publicationInformed, voluntary consent was obtained from the included patient for use of the information and materials (such as photograph) included in this review.
Copyright© The Author(s) 2022.
REFERENCES
1. Chatrath V, Banerjee PS, Goodman GJ, Rahman E. Soft-tissue filler-associated blindness: a systematic review of case reports and case series. Plast Reconstr Surg Glob Open 2019;7:e2173.
2. Liu YC, Tsai MF, Chen YF. Near complete recovery of visual acuity after calcium hydroxylapatite injection-related vision loss: a case report and literature review. Ann Plast Surg 2020;84:S123-7.
3. Master M. Hyaluronic acid filler longevity and localization: magnetic resonance imaging evidence. Plast Reconstr Surg 2021;147:50e-3e.
4. RADIESSE®. Mechanism of Action. Meamy Aesthetics 2021. Available from: https://radiesse.com/hcp/professionals/about-radiesse/how-radiesse-works/ [Last accessed on 26 Aug 2022].
5. RADIESSE®. Injectable implant instructions for use. Franksville, WI: Merz North America Inc.; 2016. Available from: https://radiesse.com/app/uploads/EM00543-04.pdf. [Last accessed on 26 Aug 2022].
6. Jacovella PF. Use of calcium hydroxylapatite (Radiesse) for facial augmentation. Clin Interv Aging 2008;3:161-74.
8. King M, Walker L, Convery C, Davies E. Management of a vascular occlusion associated with cosmetic injections. J Clin Aesthet Dermatol 2020;13:E53-8.
9. Rauso R, Sesenna E, Fragola R, Zerbinati N, Nicoletti GF, Tartaro G. Skin necrosis and vision loss or impairment after facial filler injection. J Craniofac Surg 2020;31:2289-93.
10. Page MJ, McKenzie JE, Bossuyt PM, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ 2021;372:n71.
11. Sung MS, Kim HG, Woo KI, Kim YD. Ocular ischemia and ischemic oculomotor nerve palsy after vascular embolization of injectable calcium hydroxylapatite filler. Ophthalmic Plast Reconstr Surg 2010;26:289-91.
12. Czyz C, Allen SH. Injection filler, vascular occlusion, and tissue necrosis. Otolaryngol Head Neck Surg 2011;145:P139-P139.
13. Kim YJ, Choi KS. Bilateral blindness after filler injection. Plast Reconstr Surg 2013;131:298e-9e.
14. Chang TY, Pan SC, Huang YH, Hsueh YY. Blindness after calcium hydroxylapatite injection at nose. J Plast Reconstr Aesthet Surg 2014;67:1755-7.
15. Hsiao SF, Huang YH. Partial vision recovery after iatrogenic retinal artery occlusion. BMC Ophthalmol 2014;14:120.
16. Chou CC, Chen HH, Tsai YY, Li YL, Lin HJ. Choroid vascular occlusion and ischemic optic neuropathy after facial calcium hydroxyapatite injection - a case report. BMC Surg 2015;15:21.
17. Hsieh YH, Lin CW, Huang JS, Yeh PT. Severe ocular complications following facial calcium hydroxylapatite injections: two case reports. Taiwan J Ophthalmol 2015;5:36-9.
18. Cohen E, Yatziv Y, Leibovitch I, et al. A case report of ophthalmic artery emboli secondary to Calcium Hydroxylapatite filler injection for nose augmentation - long-term outcome. BMC Ophthalmol 2016;16:98.
19. Glass LR, Choi CJ, Lee NG. Orbital complication following calcium hydroxylapatite filler injection. Ophthalmic Plast Reconstr Surg 2017;33:S16-7.
20. Marumo Y, Hiraoka M, Hashimoto M, Ohguro H. Visual impairment by multiple vascular embolization with hydroxyapatite particles. Orbit 2018;37:165-70.
21. Sung WI, Tsai S, Chen LJ. Ocular complications following cosmetic filler injection. JAMA Ophthalmol 2018;136:e180716.
22. Vu PQ, Grob SR, Tao JP. Light perception vision recovery after treatment for calcium hydroxylapatite cosmetic filler-induced blindness. Ophthalmic Plast Reconstr Surg 2018;34:e189-92.
23. Oh DJ, Jiang Y, Mieler WF. Ophthalmic artery occlusion and subsequent retinal fibrosis from a calcium hydroxylapatite filler injection. J Vitreoretin Dis 2019;3:190-3.
24. Georgescu D, Jones Y, McCann JD, Anderson RL. Skin necrosis after calcium hydroxylapatite injection into the glabellar and nasolabial folds. Ophthalmic Plast Reconstr Surg 2009;25:498-9.
26. Dayan SH, Arkins JP, Mathison CC. Management of impending necrosis associated with soft tissue filler injections. J Drugs Dermatol 2011;10:1007-12.
27. Beer K, Downie J, Beer J. A treatment protocol for vascular occlusion from particulate soft tissue augmentation. J Clin Aesthet Dermatol 2012;5:44-7.
28. Darling MD, Peterson JD, Fabi SG. Impending necrosis after injection of hyaluronic acid and calcium hydroxylapatite fillers: report of 2 cases treated with hyperbaric oxygen therapy. Dermatol Surg 2014;40:1049-52.
29. Tracy L, Ridgway J, Nelson JS, Lowe N, Wong B. Calcium hydroxylapatite associated soft tissue necrosis: a case report and treatment guideline. J Plast Reconstr Aesthet Surg 2014;67:564-8.
30. Schuster B. Injection rhinoplasty with hyaluronic acid and calcium hydroxyapatite: a retrospective survey investigating outcome and complication rates. Facial Plast Surg 2015;31:301-7.
31. Dominguez S, Moshrefi S, Dobke M. Treatment protocol for acute arterial occlusion secondary to facial revolumization procedures. Emerg Med 2017;49:221-9.
32. Rocha ACNA & Hirano CF. Extravascular occlusion with calcium hydroxyapatite filler efficiently treated with hyaluronidase. Poster presented at: 24th World Congress of Dermatology; 2019. doi:10.13140/RG.2.2.29506.73923.
33. Uittenbogaard D, Lansdorp CA, Bauland CG, Boonstra O. Hyperbaric oxygen therapy for dermal ischemia after dermal filler injection with calcium hydroxylapatite: a case report. Undersea Hyperb Med 2019;46:207-10.
34. Schelke L, Decates T, Kadouch J, Velthuis P. Incidence of vascular obstruction after filler injections. Aesthet Surg J 2020;40:NP457-60.
35. van Loghem J, Funt D, Pavicic T, et al. Managing intravascular complications following treatment with calcium hydroxylapatite: an expert consensus. J Cosmet Dermatol 2020;19:2845-58.
36. Williams MN, Burgess C. Management of calcium hydroxyapatite vascular occlusion in a hemophiliac with HIV-associated facial lipoatrophy. Dermatol Surg 2021;47:1173-4.
37. Hwang CJ, Morgan PV, Pimentel A, Sayre JW, Goldberg RA, Duckwiler G. Rethinking the role of nitroglycerin ointment in ischemic vascular filler complications: an animal model with ICG imaging. Ophthalmic Plast Reconstr Surg 2016;32:118-22.
38. thiosulfate injection dissolves calcium hydroxylapatite particles: an animal study. J Am Acad Dermatol 2018;79:AB265.
39. Robinson DM. In vitro analysis of the degradation of calcium hydroxylapatite dermal filler: a proof-of-concept study. Dermatol Surg 2018;44 Suppl 1:S5-9.
40. Danysz W, Nowag B, Hengl T, et al. Can sodium thiosulfate act as a reversal agent for calcium hydroxylapatite filler? Clin Cosmet Investig Dermatol 2020;13:1059-73.
41. Yankova M, Pavicic T, Frank K, et al. Intraarterial degradation of calcium hydroxylapatite using sodium thiosulfate - an in vitro and cadaveric study. Aesthet Surg J 2021;41:NP226-36.
42. DeLorenzi C. Complications of injectable fillers, part 2: vascular complications. Aesthet Surg J 2014;34:584-600.
43. Sclafani AP, Fagien S. Treatment of injectable soft tissue filler complications. Dermatol Surg 2009;35 Suppl 2:1672-80.
44. Lazzeri D, Agostini T, Figus M, Nardi M, Pantaloni M, Lazzeri S. Blindness following cosmetic injections of the face. Plast Reconstr Surg 2012;129:995-1012.
45. Carruthers JDA. Commentary on: a guide to doppler ultrasound analysis of the face in cosmetic medicine. Aesthet Surg J 2021;41:NP1645-6.
46. Velthuis PJ, Jansen O, Schelke LW, et al. A guide to doppler ultrasound analysis of the face in cosmetic medicine. part 2: vascular mapping. Aesthet Surg J 2021;41:NP1633-44.
47. Loghem JAJ, Humzah D, Kerscher M. Cannula versus sharp needle for placement of soft tissue fillers: an observational cadaver study. Aesthet Surg J 2017;38:73-88.
48. Yeh LC, Fabi SG, Welsh K. Arterial penetration with blunt-tipped cannulas using injectables: a false sense of safety? Dermatol Surg 2017;43:464-7.
49. Pavicic T, Webb KL, Frank K, Gotkin RH, Tamura B, Cotofana S. Arterial wall penetration forces in needles versus cannulas. Plast Reconstr Surg 2019;143:504e-12e.
50. Kim J. Novel forehead augmentation strategy: forehead depression categorization and calcium-hydroxyapatite filler delivery after tumescent injection. Plast Reconstr Surg Glob Open 2018;6:e1858.
51. Casabona G. Blood aspiration test for cosmetic fillers to prevent accidental intravascular injection in the face. Dermatol Surg 2015;41:841-7.
52. Loghem JA, Fouché JJ, Thuis J. Sensitivity of aspiration as a safety test before injection of soft tissue fillers. J Cosmet Dermatol 2018;17:39-46.
53. Cugati S, Varma DD, Chen CS, Lee AW. Treatment options for central retinal artery occlusion. Curr Treat Options Neurol 2013;15:63-77.
54. Seethapathy H, Brandenburg VM, Sinha S, El-Azhary RA, Nigwekar SU. Review: update on the management of calciphylaxis. QJM 2019;112:29-34.
55. Kirby JP, Snyder J, Schuerer DJE, Peters JS, Bochicchio GV. Essentials of hyperbaric oxygen therapy: 2019 review. Mo Med 2019;116:176-9.
56. Dhaliwal A, Gupta M. PDE5 inhibitors. [Updated 2021 Jun 25]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2022. Available from: https://www.ncbi.nlm.nih.gov/books/NBK549843/ [Last accessed on 26 Aug 2022].
57. Hew MR, Gerriets V. Prostaglandin E1. [Updated 2021 Apr 17]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2022. Available from: https://www.ncbi.nlm.nih.gov/books/NBK546629/?report=classic [Last accessed on 26 Aug 2022].
58. Khan KM, Patel J, Schaefer TJ. Nifedipine. [Updated 2021 Aug 11]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2022. Available from: https://www.ncbi.nlm.nih.gov/books/NBK537052/ [Last accessed on 26 Aug 2022].
59. Rumelt S, Dorenboim Y, Rehany U. Aggressive systematic treatment for central retinal artery occlusion. Am J Ophthalmol 1999;128:733-8.
60. Mac Grory B, Schrag M, Biousse V, et al. Management of central retinal artery occlusion: a scientific statement from the american heart association. Stroke 2021;52:e282-94.
61. Prado G, Rodríguez-Feliz J. Ocular pain and impending blindness during facial cosmetic injections: is your office prepared? Aesthetic Plast Surg 2017;41:199-203.
62. Feltgen N, Neubauer A, Jurklies B, et al. Multicenter study of the European Assessment Group for Lysis in the Eye (EAGLE) for the treatment of central retinal artery occlusion: design issues and implications. EAGLE Study report no. 1: EAGLE study report no. 1. Graefes Arch Clin Exp Ophthalmol 2006;244:950-6.
Cite This Article
Export citation file: BibTeX | RIS
OAE Style
Lindgren AL, Welsh KM. Management of vascular complications following calcium hydroxylapatite filler injections: a systemic review of cases and experimental studies. Plast Aesthet Res 2022;9:50. http://dx.doi.org/10.20517/2347-9264.2022.09
AMA Style
Lindgren AL, Welsh KM. Management of vascular complications following calcium hydroxylapatite filler injections: a systemic review of cases and experimental studies. Plastic and Aesthetic Research. 2022; 9: 50. http://dx.doi.org/10.20517/2347-9264.2022.09
Chicago/Turabian Style
Lindgren, Aleksandra L., Kathleen M. Welsh. 2022. "Management of vascular complications following calcium hydroxylapatite filler injections: a systemic review of cases and experimental studies" Plastic and Aesthetic Research. 9: 50. http://dx.doi.org/10.20517/2347-9264.2022.09
ACS Style
Lindgren, AL.; Welsh KM. Management of vascular complications following calcium hydroxylapatite filler injections: a systemic review of cases and experimental studies. Plast. Aesthet. Res. 2022, 9, 50. http://dx.doi.org/10.20517/2347-9264.2022.09
About This Article
Special Issue
Copyright
Data & Comments
Data

 Cite This Article 7 clicks
Cite This Article 7 clicks











Comments
Comments must be written in English. Spam, offensive content, impersonation, and private information will not be permitted. If any comment is reported and identified as inappropriate content by OAE staff, the comment will be removed without notice. If you have any queries or need any help, please contact us at support@oaepublish.com.