Management of symptomatic neuromas: a narrative review of the most common surgical treatment modalities in amputees
Abstract
Symptomatic neuromas are an all-too-common complication following limb amputation or extremity trauma, leading to chronic and debilitating pain for patients. Surgical resection of symptomatic neuromas has proven to be the superior method of intervention, but traditional methods of neuroma resection do not address the underlying pathophysiology leading to the formation of a future symptomatic neuroma and lead to high reoperation rates. Novel approaches employ the physiology of peripheral nerve injury to harness the regeneration of nerves to their advantage. This review explores the underlying pathophysiology of neuroma formation and centralization of pain signaling. It compares the traditional surgical approach for symptomatic neuroma resection and describes three novel surgical strategies that harness this pathophysiology of neuroma formation to their advantage. The traditional resection of symptomatic neuromas is currently the standard of care for amputation patients, but new techniques including the regenerative peripheral nerve interface, targeted muscle reinnervation, and intraosseous transposition have shown promise in improving patient pain outcomes for postamputation pain and residual limb pain. Symptomatic neuromas are a chronic and debilitating complication following amputation procedures and trauma, and the current standard of care does not address the underlying pathophysiology leading to the formation of the neuroma. New techniques are under development that may provide improved patient pain outcomes and a higher level of care for symptomatic neuroma resection.
Keywords
INTRODUCTION
There are over 2 million Americans living with major limb loss in the United States alone, with this number expected to double by 2050[1,2]. Limb amputations are the leading cause of major limb loss and are often a devastating and debilitating life event that may lead to permanent effects on a patient’s career, personal relations, and self-identity[3-5]. Amputations are more common in the lower extremity and are often indicated due to underlying vascular disease (54%), trauma (45%), or cancer (< 2%)[6]. Although amputations may provide life-saving and curative medical management for an underlying condition, these major operations are not without complications[7]. Peripheral nerve injury due to nerve transection at the time of surgery creates a proximal nerve with interrupted continuity that is no longer able to communicate with its distal innervating targets[8]. This can contribute to postamputation pain, which is perhaps one of the most prominent side effects following limb amputation and affects up to 95% of patients undergoing this procedure[9]. Postamputation pain can be categorized as phantom sensations, phantom limb pain, or residual limb pain, all of which arise due to complex signaling between the healing nerves in the residual limb and central nervous system[9]. In many cases, regeneration of the nerves that were transected during the amputation develops into a symptomatic neuroma, which has been shown to be the leading cause of residual limb pain[10,11]. This generation of symptomatic neuromas and their underlying pathophysiology has been well-described in the literature[10,11]. Moreover, a recent prospective cohort study demonstrated significant improvement in residual limb pain and phantom limb pain of amputation patients following surgical resection of their symptomatic neuroma[12]. The goal of this narrative review is to (1) summarize the underlying pathophysiology of neuroma formation and centralization of pain signaling; (2) discuss the traditional methods of surgical neuroma treatment and prevention; and (3) describe three novel surgical strategies that harness the pathophysiology of neuroma formation to their advantage.
PATHOPHYSIOLOGY OF NEUROMA GENERATION
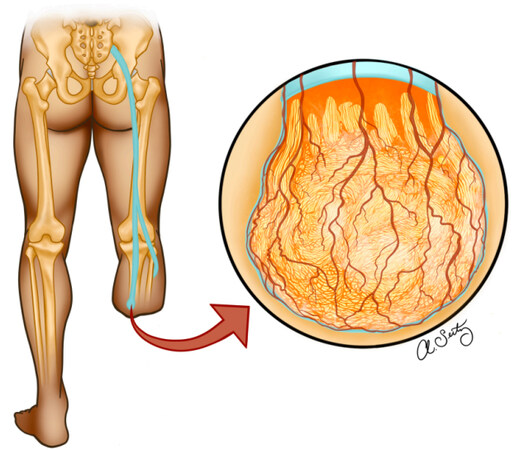
Unlike neurons of the central nervous system, nerves of the peripheral nervous system have the capacity to regenerate and create new synapses even after the most severe injury[8]. This regeneration can provide physiologic healing but, unfortunately, often occurs in a disorganized manner that leads to pathologic dysfunction of the nerve instead[8]. Central to this process are Schwann cells that are responsible for initiating and sustaining nerve regeneration[8]. In the setting of trauma, these cells dedifferentiate, proliferate, and elongate within the endoneurial tubes to help guide the reconnection of a transected nerve back into its distal nerve sheath[8]. Schwann cells release hundreds of growth-associated genes that trigger a nerve fiber to switch from a transmitting mode into a growth mode. This signaling cascade begins the process of axonal growth, which is marked by the production of a growth cone at a fiber’s last healthy node of Ranvier[8]. Unfortunately, in amputation patients, the transected nerve will never reconnect with its distal sheath and will continue its search for the sheath as long as it remains in the regenerative state. Proximal axons randomly sprout from the proximal nerve with hopes of establishing a connection with the lost distal nerve. To facilitate and sustain this growth, new connective tissue and blood vessels within the epineurium, perineurium, and endoneurium are produced surrounding these growing axons[13]. Over time, the disorganized growth of axons, connective tissue, and blood vessels form a neurovascular sphere at the proximal nerve ending, which is collectively termed a neuroma [Figure 1][13].
Figure 1. Illustration of neurovascular sphere growth following tibial nerve transection, leading to the eventual production of a neuroma. Illustrated by Seitz AJ.
Neuromas are fundamentally different than healthy peripheral nerve tissue in both structure and composition. As axons continue to grow without an intact endoneurial tube or distal target to guide them, axons may innervate surrounding targets sporadically, such as the skin, which allows for propagation of action potentials through anomalous pathways[14]. Neuromas also express different transduction molecules, upregulate sodium channels, and downregulate potassium channels embedded within the cell membranes of axons[14]. External stimuli such as cytokines and macrophages also trigger myofibroblast formation and proliferation at the site of a neuroma causing contraction of scar tissue which in turn further aggravates the disorganized bundle of nerves[8]. These changes all contribute to creating hyperexcitable neural tissue that can spontaneously discharge and produce inappropriate afferent pain signals for a patient[14]. Over time, these signals often lead to central nervous system imbalances between excitatory and inhibitory signaling pathways, amplifying the intensity of each subsequent pain signal; this process is known as central sensitization[2]. From a clinical standpoint, a cross-sectional study that was conducted on 124 military veterans with traumatic amputation injury showed that 64.5% of service members had clinically significant pain following the amputation procedure, with 48.7% of these patients’ pain directly attributed to symptomatic and sensitized neuroma formation[15]. Although the growth of axons in the residual nerve is considered physiologic in the regeneration of a peripheral nerve, the downstream effects have often shown to manifest as pathologic pain [Figure 2].
TRADITIONAL TREATMENTS OF SYMPTOMATIC NEUROMAS
With such a high percentage of patients experiencing painful consequences of symptomatic neuromas, a myriad of treatment options have been explored to help alleviate this pain. Conservative strategies such as desensitization therapy, electrical biofeedback devices, anesthetic injections, and pharmacotherapy with antidepressants, anticonvulsants, and opioids have all been attempted with varying degrees of short-term success[13,16]. Although these treatment modalities may be less invasive, surgical intervention of symptomatic neuromas has shown to provide superior pain management. A meta-analysis comparing surgical interventions for symptomatic neuroma treatment found that 77% of patients undergoing neuroma resection had significant improvement in patient-reported pain, depression, and quality of life shortly after surgery regardless of the surgical technique used[17]. However, even with this seemingly high initial success rate, 20%-30% of symptomatic neuromas remain refractory to surgery, and reoperation rates for all symptomatic neuromas have been observed to be as high as 65%[17]. Although traditional surgical approaches provide initial symptomatic relief, pathologic pain returns all too often.
Excision under tension with retraction into soft tissue
The most common approach for the removal of a neuroma is simple neuroma excision[7]. In this approach, the neuroma is visualized and dissected away from surrounding tissue before transecting the involved nerve to resect the neuroma. As one might anticipate, this freshly transected nerve will undergo the same regenerative sprouting and axonal elongation that caused the formation of the original neuroma. To mitigate this, a surgeon can transect the nerve under tension to allow the nerve to recoil deep into a muscle belly. Less commonly, a surgeon may also elect to manually redirect the transected nerve and suture it into a neighboring muscle[7]. Burying the end of the transected nerve into muscle through either of these methods allows the inevitable future neuroma to relocate to a deeper and more protected space, away from any irritating external stimuli[7]. Moreover, any anomalous innervation and interaction with adjacent tissue are far away from problem structures such as the skin which decreases the chances of a hypersensitive symptomatic neuroma[18].
The technique of relocating the residual nerve into a muscle belly is the most common neuroma resection approach and dates back to 1918 when it was successfully done on two patients[16]. Today, this technique is still commonly used and completed prophylactically during amputation procedures to reduce the risk of future symptomatic neuroma formation[16]. Allowing the transected nerve to be buried into the surrounding muscles has shown positive initial results for pain relief, with a clinical study involving 60 patients with symptomatic neuromas showing 81% of patients reporting reduced pain following surgery at 31 months postoperatively[19]. However, this same study showed that success rate is highly dependent on neuroma location and presentation[19]. Neuromas in regions of the body that have large, secluded muscle bellies to bury the transected nerve tend to have the best results, such as the forearm or thigh. Regions of the body that do not have deep muscle bellies, such as the palm or digits of the upper extremity, showed success rates of 14% with this approach[19].
The simple neuroma excision with nerve retraction or manual burial into muscle relies on creating a safe and privileged environment to shield the inevitable future neuroma from external stimuli. Although this approach is used as standard therapy to reduce the risk of symptomatic neuroma formation, it does not address the pathologic formation of a neuroma which is likely why the reoperation rates with this technique remain as high as 65%[17].
TARGETED MUSCLE REINNERVATION
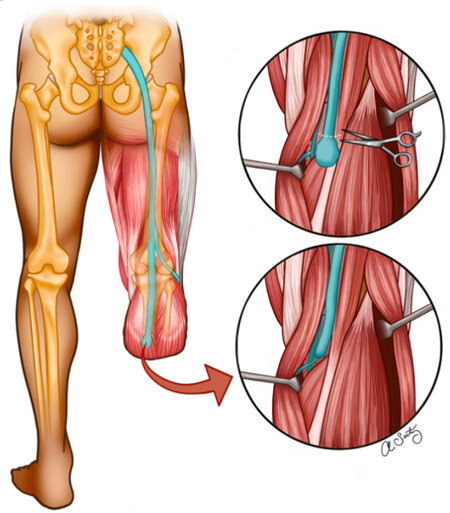
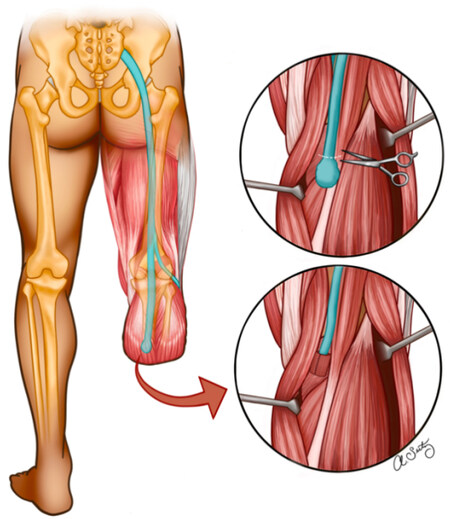
Targeted muscle reinnervation (TMR) was originally developed in 2002 as a method to augment the interface of myoelectric prosthetics but was soon expanded for the treatment of symptomatic neuromas[20,21]. The basis of this surgical approach is to introduce an autologous denervated target, in the form of skeletal muscle, that has a high propensity for reinnervation in order to guide the newly transected nerve into growing and establishing a connection with this target. TMR is considered a nerve transfer because it transects a healthy motor nerve near the neuroma site to create the denervated skeletal muscle target for the residual nerve to reinnervate [Figure 3][22]. The mixed nerve’s motor neurons then grow into the denervated skeletal muscle and form new synapses, halting the growth of the neurons[2]. By providing a target for the transected nerve to reinnervate, TMR provides the regenerating nerve “somewhere to go and something to do”, a term coined by Souza et al.[23]. In doing so, TMR harnesses the physiology of a regenerating nerve and works to prevent the pathologic formation of a future neuroma.
Figure 3. Illustration of the TMR surgical approach. Tibial nerve neuroma is resected, followed by denervation of the medial head of the gastrocnemius muscle motor nerve. Subsequent anastomosis of the tibial nerve to the medial gastrocnemius muscle. Illustrated by Seitz AJ. TMR: Targeted muscle reinnervation.
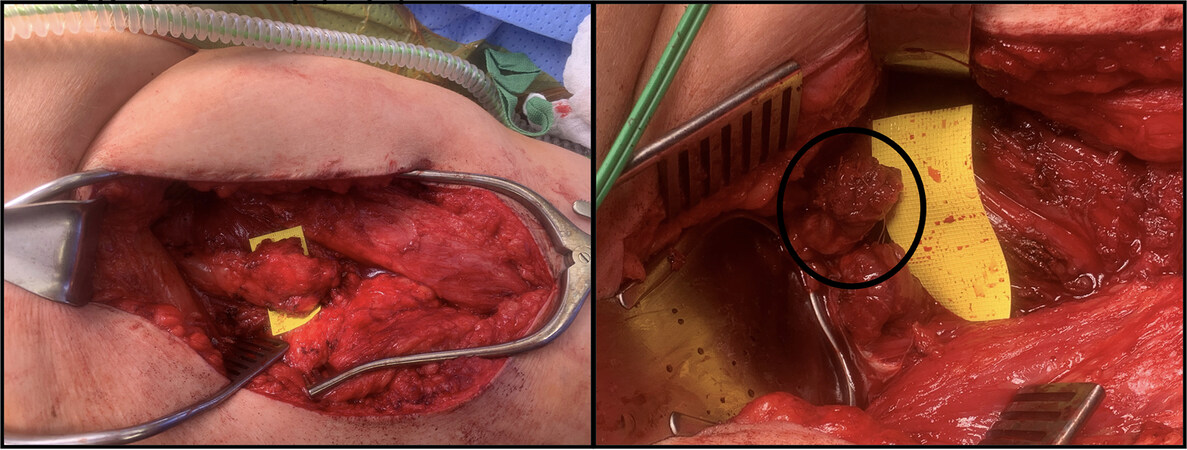
The surgical approach of TMR involves isolation of the symptomatic neuroma and proximal transection of the involved nerve to resect the neuroma. The transected nerve is dissected away from surrounding tissue and sharply cut to expose all of the nerve’s fascicles, preserving as much length as possible[22]. Next, an electric nerve stimulator is utilized to locate and isolate motor nerves that are innervating healthy skeletal muscle adjacent to the site of the residual nerve[22]. Muscles are specifically sought out that are functionally not required or redundant to preserve the utility of an amputated limb[18]. Once the motor nerve is identified with a nerve stimulator, the motor nerve is transected near its entry into the muscle, thereby creating a denervated muscle target[22]. The residual nerve is then transferred to the motor nerve’s muscle entry site and epineurial anastomosis is performed[22]. Ideally, a small region of skeletal muscle around this site is also dissected away and folded over the anastomosis to suture to the nerve epineurium in order to optimize reinnervation of the muscle [Figure 4][22]. Although the TMR procedure results in a newly transected pure motor nerve in order to create a target for the mixed nerve, this transected pure motor nerve has not been shown to produce a clinically symptomatic neuroma[20].
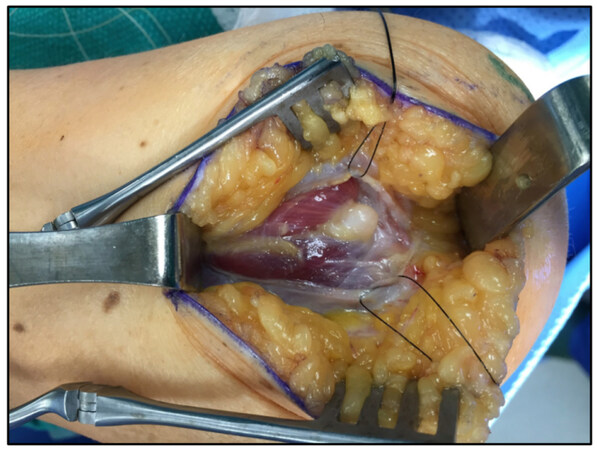
Figure 4. Sciatic nerve neuroma resection (left) with differentiation of 4 fascicles. TMR involving fascicles of the sciatic nerve (right) with an anastomotic connection to adjacent muscle neuron entry into muscle. Photo credit to Dr. Poore SO. TMR: Targeted muscle reinnervation.
Preclinical trials of TMR include an animal study involving five rabbits with neuromas, each undergoing TMR procedures on their median, ulnar, and radial nerves onto motor nerves of their rectus abdominis muscles[24]. Ten weeks following surgery, histologic analysis of all of the anastomosis sites showed partially regenerated nerves without the formation of a new neuroma, while electromyography showed partial reinnervation of the rectus abdominis muscles in a segmental fashion[24]. The histologic analysis also showed decreased myelination of the nerves and increased fascicle diameter, both of which are considered favorable characteristics following neuroma resection[24]. Thus, this investigation was able to show successful reinnervation of skeletal muscles using mixed nerves and prevention of neuroma formation using the TMR approach[24].
Following these preclinical trials, Dumanian et al. compared TMR to standard simple neuroma excision with muscle burial in 28 patients[20]. Patients were blinded and followed postoperatively over a 1-year period. Results revealed an overall significant improvement in patients’ phantom limb pain and a trend for improvement in a patient’s residual limb pain[20]. Although the TMR approach may provide a more comprehensive approach to the treatment and prevention of neuroma formation, further and more longitudinal investigations both at the preclinical and clinical levels must be conducted to illustrate its efficacy over other methods.
REGENERATIVE PERIPHERAL NERVE INTERFACES
Similar to TMR, the regenerative peripheral nerve interface (RPNI) was designed as a methodology that could augment and terminate a nerve’s search for reinnervation by providing an alternative target for the newly transected nerve[25]. RPNI was originally developed as a bridge for amputated limbs to be able to communicate with neuro-prosthetic devices, but quickly evolved into a technique to treat symptomatic neuromas[26]. This surgical approach introduces an autologous denervated target in the form of a skeletal muscle graft, which has a high propensity for reinnervation in order to guide the newly transected nerve into growing and establishing a connection with this target. For prosthetic control, an implantable electrode is placed within the reinnervated muscle graft which serves as a signal amplifier in order to communicate with an external prosthetic device. Similar to the TMR approach, the motor neurons of the transected nerve grow into and reinnervate the skeletal muscle graft, switching from growth mode back into transmission[2]. By providing a target for the transected nerve to reinnervate, RPNI provides the regenerating nerve somewhere to go and something to do, and harnesses the physiology of a regenerating nerve to prevent the pathologic formation of a future neuroma [Figure 5].
Figure 5. Illustration of the regenerative peripheral nerve interface approach. A tibial neuroma is resected, followed by anastomosis of the proximal nerve end with a skeletal muscle graft, most likely taken from the vastus lateralis muscle. Skeletal muscle fibers are fastened parallel to the nerve fibers and buried deep underneath the adjacent muscles of the leg. Illustrated by Seitz AJ.
The first stage of RPNI surgery is the same as a simple neuroma excision, with isolation of the neuroma and proximal transection of the involved nerve to resect the neuroma. Then, the newly transected nerve is dissected away from its surrounding tissue, and the proximal transection site is made to be a sharp cut with all of the nerve’s fascicles exposed[27]. Once the neuroma is excised and the freshly cut nerve-end is isolated, the nerve is implanted into a free skeletal muscle graft[2]. If the RPNI is done during an amputation procedure, the skeletal muscle graft is harvested from the distal amputated limb to preserve healthy tissue. If the RPNI is done after the amputation procedure, the skeletal muscle graft is most commonly obtained from a neighboring skeletal muscle (e.g., the vastus lateralis muscle in the case of lower extremity amputation). Small grafts carefully harvested from the muscle result in an insignificant alteration to the muscle’s function[2]. Regardless of the harvest site, it is important to note that these skeletal muscle grafts are severed from their blood supply and innervation, while the skeletal muscle in the TMR approach maintains its blood supply. The grafts are harvested as small cubes that are cut parallel to the axis of the muscle fibers to ensure a maximal number of intact fibers that retain the capacity to be reinnervated[27]. The skeletal muscle grafts are created as cubes, typically 30-40 mm long, 15-20 mm wide, and 5-6 mm thick[28]. Larger nerves such as a sciatic nerve may need a large number of muscle fibers to provide an adequate number of reinnervation targets. In these cases, it is appropriate to dissect the large nerve into multiple fascicular branches and use multiple muscle grafts for RPNIs[27].
The second stage of the RPNI surgery is focused on attaching the harvested skeletal muscle graft onto the newly transected nerve. The skeletal muscle graft is brought to the transected nerve and placed so that the nerve is in the center of the graft and parallel to its muscle fibers[27]. The epineurium of the nerve is then sutured into the graft using 2 6-0 non-absorbable monofilament sutures before the edges of the muscle graft are then gently wrapped circumferentially around the nerve to encompass the transected end and secured using the same monofilament sutures[27]. This nerve-graft bundle is then buried deep into a bluntly dissected muscle belly that is away from any weight-bearing surface and surgical incision site before closing the surgical site [Figure 6][27].
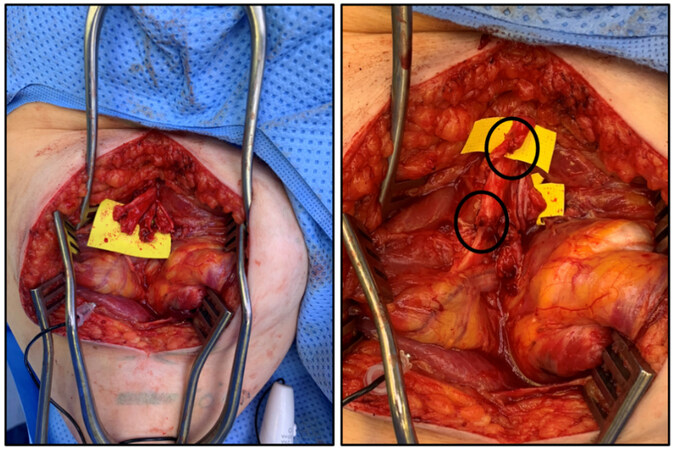
Figure 6. (left) Recurrent symptomatic neuroma of sciatic nerve following TMR surgery (same patient as Figure 4). (right) Patient undergoing reoperation with regenerative peripheral nerve interface following resection of recurrent sciatic nerve neuroma. Skeletal muscle graft fastened and wrapped around the nerve ending prior to deep muscle burial of RPNI. Photo credit to Dr. Poore SO. TMR: Targeted muscle reinnervation; RPNI: regenerative peripheral nerve interface.
Multiple animal studies have shown evidence of angiogenesis and revascularization of the muscle graft, as well as the formation of new neuromuscular junctions between the transected nerve and muscle fibers[29,30]. Moreover, compared to simple neuroma excision, these animal studies have shown that there is an absence of symptomatic neuroma formation with the RPNI approach[31]. These promising preclinical studies propelled the use of the RPNI approach in human patients starting in 2013 at the University of Michigan, with over 250 patients receiving this treatment since then[28].
In a retrospective review of 90 patients, RPNI surgery has shown a significant decrease in symptomatic neuroma pain (0% vs. 13.3%) when done prophylactically at the time of amputation compared to simple neuroma excision with burial in muscle[28]. Moreover, these data also support an overall significant decrease in phantom limb pain (51.1% vs. 91.1%) and post-operative complication rate (31.1% vs. 55.6%) at 1year follow-up[28]. Although RPNI surgery has shown positive results in both animal studies and clinical trials, drawbacks of this approach include an increased operative time by 8-12 min per muscle graft, as well as the possibility of hematoma formation at the muscle tissue harvest site[27]. Further longitudinal studies will allow for a more complete understanding of the long-term benefits and consequences RPNI may have for symptomatic neuroma formation as well as control of neural prosthetics.
INTRAOSSEOUS TRANSPOSITION
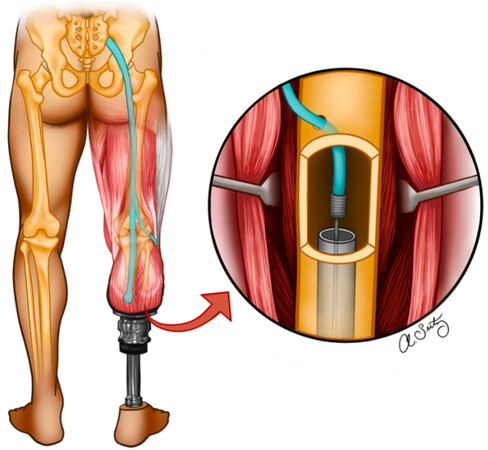
Unlike the TMR and RPNI approaches, an intraosseous (IO) transposition relocates a transected nerve into bone rather than muscle. This approach was first reported in 1943 when a transected nerve was buried into the spongy bone and thus protected from external stimuli by the encasing bone[32]. More recently, this technique was again reported as a viable methodology for the treatment of symptomatic neuromas transposing nerves into the medullary cavity of long bones[1]. Creating a transposition into the medullary cavity of a long bone instead of spongy bone affords a much larger and protected space for the residual nerve and also allows for larger nerves to be transposed. This technique has also recently been shown as a methodology for neural interfacing by utilizing an electrode interface on the residual nerve placed within the medullary canal[33]. This technology - the Osseointegrated Neural Interface (ONI) - provides a stable platform that is bimodal, including the treatment and prevention of amputation pain and the creation of a neural interface for prosthesis control [Figure 7][33].
Figure 7. Illustration of the intraosseous transposition of a resected tibial nerve neuroma into the tibial medullary cavity. Photo includes a potential intramedullary electrode for an Osseointegrated neural interface. Illustrated by Seitz AJ.
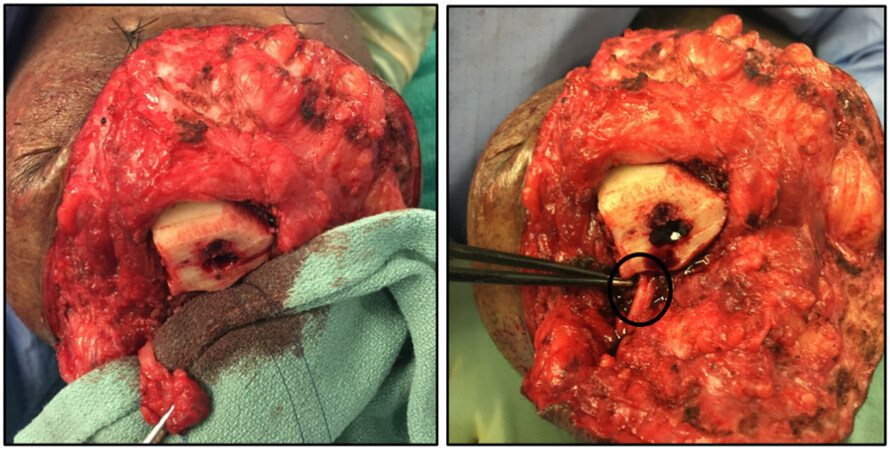
The IO transposition approach begins in the same fashion as the previous techniques, with isolation and resection of the symptomatic neuroma and proximal transection of the involved nerve. Next, the underlying bone is identified, and a cortical surface is cleared for creating a corticotomy. The nerve is then passed into the canal and the perineurium is secured to the periosteum with a small diameter nylon suture before closing the incision site [Figure 8].
Figure 8. Intraosseous transposition following tibial nerve neuroma (left) resection with tibial corticotomy and implantation of the proximal transected nerve into the medullary cavity (right). Photo credit to Dr. Poore SO.
Clinical studies of IO transposition into spongy bone without ONI have shown a 70% success rate in patients with symptomatic neuromas of the hand[34]. IO transpositions have also been used in patients with hand neuromas who have failed their first attempt at surgical intervention, with a case report of 11 patients reporting a success rate after repeat surgery in 10 of the patients with a mean follow-up of 25 months[35]. Although intraosseous transpositions have shown high rates of success in burying in bone, this approach has been limited to use mainly within the hand and is not without complications. Common complications, including missing branches of a transected nerve, and the possibility of the buried nerve being pulled out of the bone and back into the environment, lead to exacerbation of symptoms and the need for repeat surgery[35]. Conversely, IO transposition into the medullary cavity of long bones has shown excellent results, with two case studies involving a tibial nerve implanted into the tibia and a sciatic nerve implanted into the femur showing complete resolution of the patients’ debilitating pain[1]. However, this approach is novel and has not been tested extensively in larger clinical trials. Further investigation of this method is needed to better understand the long-term sequelae and recurrence of symptomatic neuroma formation.
IO transposition with ONI is currently in preclinical trials, with the first ONI completed involving a rabbit[36] for proof of concept and a follow-up study showing efficacy using a more complex sieve electrode[33]. Although this stable and more complex interface has shown promise, further animal studies are needed to assess the efficacy of the IO transposition with ONI in creating stable, high-fidelity interfaces.
DISCUSSION
The surgical management of symptomatic neuromas has evolved over the past two decades due to a deepening understanding of peripheral nerve injury and repair. The RPNI and TMR techniques were not developed with the intention of better outcomes of neuroma resection but were rather discovered during the development of higher-quality neuro-prosthetics and peripheral nerve interfaces[20,25]. The IO transposition was originally designed for the treatment of symptomatic neuroma and has since been extended as a platform for neural interfacing within a protected and secluded environment[1]. The traditional approach to resect a neuroma and bury the transected nerve into a muscle belly or bone has been the standard of care for decades, but this approach fails to address the underlying regenerative changes of a transected nerve that lead to the creation of a symptomatic neuroma. Hiding the residual nerve deep within the muscle may provide additional shielding but will inevitably lead to the production of a future neuroma, which is likely why 20%-30% of symptomatic neuromas are refractory to this approach and reoperation rates are as high as 65%[17].
The TMR approach was the first alternative treatment modality to this traditional approach and was originally developed as the first transcutaneous myoelectric neural interface to communicate with a prosthetic[20]. Through efforts to improve prosthetic control, TMR inadvertently addressed the underlying physiology that leads to symptomatic neuroma formation[37]. This has been clinically shown to reduce phantom limb pain and rates of opioid use in amputation patients and is now used routinely during amputation procedures as prophylaxis to prevent future neuroma formation[12,20]. However, this approach is limited by the location of the anastomosis site and the number of skeletal muscles that are denervated from their motor neurons for coaptation of the transected nerve. Moreover, the TMR approach sacrifices some of the traditional shielding benefits that come from burying a nerve into muscle or bone, which may lead to a future recurrence of a symptomatic neuroma.
Similar to TMR, the RPNI approach was also conceived as a means to improve and fine-tune neuro-prosthetic control[31]. Providing a skeletal muscle target for the severed nerve inadvertently led to significant decreases in symptomatic neuroma pain and phantom limb pain, enjoying the same benefits of TMR by addressing the underlying regenerative physiology[28]. The RPNI approach excels compared to the TMR approach in that multiple muscle grafts can be used for multiple nerves, and larger nerves can be split among multiple grafts for better size-matching[27]. Since the RPNI approach does not require transecting healthy pure motor nerves, there is no limit to the number of muscle grafts that can be used for this approach[18]. Additionally, RPNI provides enhanced flexibility in choosing targets and does not rely on the potential limitation of viable motor nerve targets. However, due to the lack of direct blood supply to the muscle grafts, the RPNI approach is limited by the size of the individual grafts and can potentially lead to avascular necrosis of the grafts if they are crafted too large[27].
The IO approach with medullary transposition offers the transected nerve a secluded and privileged environment compared to the muscle-based approaches. The medullary canal also provides an environment that is highly vascular and contains stem cells, providing an optimal environment for a transected nerve[38]. The highly shielded medullary cavity provides a safe environment far from the surface of the skin and any moving muscle, while the ONI provides a target to harness the underlying physiology of a regenerating nerve. Moreover, the larger space within the medulla allows for more complex and bidirectional electrodes that can be securely fastened to the cortical bone, which may allow future prosthetics with more selectivity and a higher-fidelity signal[18,33]. However, the IO with ONI approach has yet to be tested in a large animal model, and thus conclusions regarding the usefulness of this as a neural interface in humans must be guarded[36].
CONCLUSION
This review serves as a benchmark for assessing the current common treatment modalities of symptomatic neuromas and highlights the differences among each approach. Although the standard of care for neuroma treatment has remained stable over the past few decades, developments in the world of neuro-prosthetics have extended our understanding of peripheral nerve injury and repair and have provided alternative treatment avenues that take advantage of the underlying physiology of a regenerating nerve. The cornerstones of symptomatic neuroma resection include providing a regenerating nerve with a secluded and privileged environment and also a target to reinnervate. Following these principles have proven clinical improvement in patients’ pain and quality of life, but no treatment modality has proven to be superior to others. Further collaboration and investigation of the clinical utility of each method may lead to improved clinical outcomes for patients and may also inadvertently advance the field of neuro-prosthetics.
DECLARATIONS
Authors’ contributionsMade substantial contributions to the literature review, data acquisition, interpretation, and manuscript production: Eftekari SC, Nicksic PJ, Seitz AJ, Donnelly DT, Dingle AM, Poore SO
Production of illustrations: Seitz AJ
Availability of data and materialsNot applicable.
Financial support and sponsorshipNone.
Conflicts of interestAll authors declared that there are no conflicts of interest.
Ethical approval and consent to participateThis study has been exempt from institutional review board approval. This narrative review did not conduct research on human subjects. All clinical photos employed were provided by Dr. Samuel O. Poore in an anonymous and deidentified manner.
Consent for publicationNot applicable.
Copyright© The Author(s) 2022.
REFERENCES
1. Israel JS, Dingle AM, Sanchez RJ, et al. Neuroma implantation into long bones: clinical foundation for a novel osseointegrated peripheral nerve interface. Plast Reconstr Surg Glob Open 2018;6:e1788.
2. Santosa KB, Oliver JD, Cederna PS, Kung TA. Regenerative peripheral nerve interfaces for prevention and management of neuromas. Clin Plast Surg 2020;47:311-21.
3. Adamo MA, Pollack IF. A single-center experience with symptomatic postoperative calvarial growth restriction after extended strip craniectomy for sagittal craniosynostosis. J Neurosurg Pediatr 2010;5:131-5.
4. Resnik L, Etter K, Klinger SL, Kambe C. Using virtual reality environment to facilitate training with advanced upper-limb prosthesis. J Rehabil Res Dev 2011;48:707-18.
5. Sinha R, van den Heuvel WJ, Arokiasamy P. Factors affecting quality of life in lower limb amputees. Prosthet Orthot Int 2011;35:90-6.
6. Pinzur MS, Gottschalk FA, Pinto MA, Smith DG. American Academy of Orthopaedic Surgeons. Controversies in lower-extremity amputation. J Bone Joint Surg Am 2007;89:1118-27.
7. Sehirlioglu A, Ozturk C, Yazicioglu K, Tugcu I, Yilmaz B, Goktepe AS. Painful neuroma requiring surgical excision after lower limb amputation caused by landmine explosions. Int Orthop 2009;33:533-6.
9. Ephraim PL, Wegener ST, MacKenzie EJ, Dillingham TR, Pezzin LE. Phantom pain, residual limb pain, and back pain in amputees: results of a national survey. Arch Phys Med Rehabil 2005;86:1910-9.
10. Economides JM, DeFazio MV, Attinger CE, Barbour JR. Prevention of painful neuroma and phantom limb pain after transfemoral amputations through concomitant nerve coaptation and collagen nerve wrapping. Neurosurgery 2016;79:508-13.
11. Prantl L, Schreml S, Heine N, Eisenmann-Klein M, Angele P. Surgical treatment of chronic phantom limb sensation and limb pain after lower limb amputation. Plast Reconstr Surg 2006;118:1562-72.
12. Mioton LM, Dumanian GA, Shah N, et al. Targeted muscle reinnervation improves residual limb pain, phantom limb pain, and limb function: a prospective study of 33 major limb amputees. Clin Orthop Relat Res 2020;478:2161-7.
13. Stokvis A, van der Avoort DJC, van Neck JW, Hovius SER, Coert JH. Surgical management of neuroma pain: a prospective follow-up study. Pain 2010;151:862-9.
15. Buchheit T, Van de Ven T, Hsia HL, et al. Pain phenotypes and associated clinical risk factors following traumatic amputation: results from veterans integrated pain evaluation research (VIPER). Pain Med 2016;17:149-61.
16. Wu J, Chiu DT. Painful neuromas: a review of treatment modalities. Ann Plast Surg 1999;43(6):661-7.
17. Poppler LH, Parikh RP, Bichanich MJ, et al. Surgical interventions for the treatment of painful neuroma: a comparative meta-analysis. Pain 2018;159:214-23.
18. Karczewski AM, Dingle AM, Poore SO. The need to work arm in arm: calling for collaboration in delivering neuroprosthetic limb replacements. Front Neurorobot 2021;15:711028.
19. Dellon AL, Mackinnon SE. Treatment of the painful neuroma by neuroma resection and muscle implantation. Plast Reconstr Surg 1986;77:427-38.
20. Dumanian GA, Potter BK, Mioton LM, et al. Targeted muscle reinnervation treats neuroma and phantom pain in major limb amputees: a randomized clinical trial. Ann Surg 2019;270:238-46.
21. Hijjawi JB, Kuiken TA, Lipschutz RD, Miller LA, Stubblefield KA, Dumanian GA. Improved myoelectric prosthesis control accomplished using multiple nerve transfers. Plast Reconstr Surg 2006;118:1573-8.
22. Bowen JB, Ruter D, Wee C, West J, Valerio IL. Targeted muscle reinnervation technique in below-knee amputation. Plast Reconstr Surg 2019;143:309-12.
23. Souza JM, Cheesborough JE, Ko JH, Cho MS, Kuiken TA, Dumanian GA. Targeted muscle reinnervation: a novel approach to postamputation neuroma pain. Clin Orthop Relat Res 2014;472:2984-90.
24. Kim PS, Ko JH, O’Shaughnessy KK, Kuiken TA, Pohlmeyer EA, Dumanian GA. The effects of targeted muscle reinnervation on neuromas in a rabbit rectus abdominis flap model. J Hand Surg Am 2012;37:1609-16.
25. Woo SL, Kung TA, Brown DL, Leonard JA, Kelly BM, Cederna PS. Regenerative peripheral nerve interfaces for the treatment of postamputation neuroma pain: a pilot study. Plast Reconstr Surg Glob Open 2016;4:e1038.
26. Kumar N, Kung TA, Cederna PS. Regenerative peripheral nerve interfaces for advanced control of upper extremity prosthetic devices. Hand Clin 2021;37:425-33.
27. Kubiak CA, Adidharma W, Kung TA, Kemp SWP, Cederna PS, Vemuri C. “Decreasing postamputation pain with the regenerative peripheral nerve interface (RPNI)”. Ann Vasc Surg 2022;79:421-6.
28. Kubiak CA, Kemp SWP, Cederna PS, Kung TA. Prophylactic regenerative peripheral nerve interfaces to prevent postamputation pain. Plast Reconstr Surg 2019;144:421e-30e.
29. Frost CM, Ursu DC, Flattery SM, et al. Regenerative peripheral nerve interfaces for real-time, proportional control of a neuroprosthetic hand. J Neuroeng Rehabil 2018;15:108.
30. Svientek SR, Ursu DC, Cederna PS, Kemp SWP. Fabrication of the composite regenerative peripheral nerve interface (C-RPNI) in the adult rat. J Vis Exp 2020; doi: 10.3791/60841.
31. Kung TA, Langhals NB, Martin DC, Johnson PJ, Cederna PS, Urbanchek MG. Regenerative peripheral nerve interface viability and signal transduction with an implanted electrode. Plast Reconstr Surg 2014;133:1380-94.
33. Dingle AM, Ness JP, Novello J, et al. Methodology for creating a chronic osseointegrated neural interface for prosthetic control in rabbits. J Neurosci Methods 2020;331:108504.
34. Mass DP, Ciano MC, Tortosa R, Newmeyer WL, Kilgore ES Jr. Treatment of painful hand neuromas by their transfer into bone. Plast Reconstr Surg 1984;74:182-5.
35. Goldstein SA, Sturim HS. Intraosseous nerve transposition for treatment of painful neuromas. J Hand Surg Am 1985;10:270-4.
36. Dingle AM, Novelo J, Ness JP, Israel JS, Poore SO. Abstract 55: osseointegrated neural interface (ONI). Plastic and Reconstructive Surgery - Global Open 2017;5:42-3.DOI:10.1097/01.GOX.0000516575.01834.51.
37. Janes LE, Fracol ME, Dumanian GA, Ko JH. Targeted muscle reinnervation for the treatment of neuroma. Hand Clin 2021;37:345-59.
Cite This Article
Export citation file: BibTeX | RIS
OAE Style
Eftekari SC, Nicksic PJ, Seitz AJ, Donnelly DAT, Dingle AM, Poore SO. Management of symptomatic neuromas: a narrative review of the most common surgical treatment modalities in amputees. Plast Aesthet Res 2022;9:43. http://dx.doi.org/10.20517/2347-9264.2022.33
AMA Style
Eftekari SC, Nicksic PJ, Seitz AJ, Donnelly DAT, Dingle AM, Poore SO. Management of symptomatic neuromas: a narrative review of the most common surgical treatment modalities in amputees. Plastic and Aesthetic Research. 2022; 9(7): 43. http://dx.doi.org/10.20517/2347-9264.2022.33
Chicago/Turabian Style
Eftekari, Sahand C., Peter J. Nicksic, Allison J. Seitz, D’Andrea T. Donnelly, Aaron M. Dingle, Samuel O. Poore. 2022. "Management of symptomatic neuromas: a narrative review of the most common surgical treatment modalities in amputees" Plastic and Aesthetic Research. 9, no.7: 43. http://dx.doi.org/10.20517/2347-9264.2022.33
ACS Style
Eftekari, SC.; Nicksic PJ.; Seitz AJ.; Donnelly DAT.; Dingle AM.; Poore SO. Management of symptomatic neuromas: a narrative review of the most common surgical treatment modalities in amputees. Plast. Aesthet. Res. 2022, 9, 43. http://dx.doi.org/10.20517/2347-9264.2022.33
About This Article
Special Issue
Copyright
Data & Comments
Data
 Cite This Article 51 clicks
Cite This Article 51 clicks












Comments
Comments must be written in English. Spam, offensive content, impersonation, and private information will not be permitted. If any comment is reported and identified as inappropriate content by OAE staff, the comment will be removed without notice. If you have any queries or need any help, please contact us at support@oaepublish.com.