Tackling bone loss of the lower extremity: vascularized bone grafting
Abstract
Post-traumatic lower extremity bone loss in the setting of high-energy trauma can occur acutely as a result of an open fracture and surgical debridement, or secondarily as a result of nonunion or infection. Several techniques have been described in the literature for the management of these bony defects, including non-vascularized bone grafts, vascularized bone grafts and distraction osteogenesis. Herein, the authors review the role of vascularized bone grafts in the management of post-traumatic bone loss in the lower extremity.
Keywords
INTRODUCTION
The first step in the management of patients with high energy trauma resulting in open fractures of the lower extremity includes early and aggressive debridement of devitalized and contaminated soft tissue and bone. Multiple operative debridements are often required before bony reconstruction can be considered[1,2]. Bone defects following lower extremity trauma occur acutely in the setting of significant fracture comminution, open fractures, and following aggressive debridement and secondarily in the setting of aseptic or septic nonunion. Temporary external fixators are used to stabilize the extremity and maintain length[3]. The goal of bony reconstruction is to provide stability and reestablish limb length.
The first successful free vascularized bone graft was performed and described by Taylor et al.[4] in 1978 for a post-traumatic intercalary bone defect - a free fibula flap was used to reconstruct a 12.5 cm defect of the tibia. Subsequently, the vascularized rib (Buncke et al.[5], 1977) and vascularized iliac crest (Taylor and Watson[6], 1978) bone grafts were successfully performed and described in the literature for lower extremity bony reconstruction. The medial femoral condyle flap was first described in 1990 by Hayashi and Maruyama, and applied to lower extremity reconstruction by Doi and Sakai[7] in 1994. Since the 1970s, the vascularized free fibula flap has remained the workhorse flap for post-traumatic lower extremity bony reconstruction, followed by the less commonly utilized iliac crest, medial femoral condyle and rib donor sites[8,9].
INDICATIONS FOR VASCULARIZED BONE GRAFTS
Several classification systems have been created in attempts to uniformly describe the degree of lower extremity trauma. The Gustilo and Anderson[10] classification is well known and categorizes open fractures based on the degree of soft tissue injury. Unlike the Gustilo system, the extent of bony defects can be described with Winquist’s classification system. This system was originally created to describe femur fractures; however, it has been subsequently applied to tibial shaft fractures[3,11]. Battiston et al.[3] recommend non-vascularized bone grafting for Winquist grade 1 or 2 fractures (no or minimal comminution and more than 50% contact between fragments with moderate comminution, respectively). For fractures with less than 50% contact between fragments, more severe comminution, or segmental bone loss (Winquist grades 3 and 4), reconstruction with bone transport or vascularized bone grafting is suggested[3]. While several techniques have been described for bony reconstruction, vascularized bone grafting remains the modality of choice in the setting of large bony defects (> 6 cm)[12,13]. Additional indications for vascularized bone grafting include poor vascularity of the recipient site, repeated failure of non-vascularized bone grafts, and fracture nonunion[14].
BIOLOGY
The proposed advantages of vascularized bone grafting for reconstruction of post-traumatic lower extremity bone defects include a sturdier immediate reconstruction and the capacity for osteogenesis, which ultimately allow for faster incorporation and primary bone union times[3,12]. Unlike avascular bone grafts, the process of creeping substitution is bypassed. Therefore, vascularized bone grafts have less resorption with better retention of bony architecture, resistance to infection, mechanical strength, and healing potential[15,16]. Additionally, vascularized bone grafts are capable of responding to applied biomechanical stresses with hypertrophy[17].
GRAFT TYPES
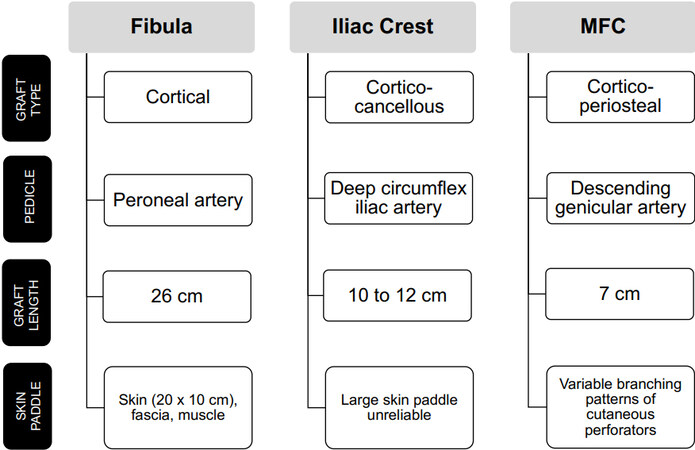
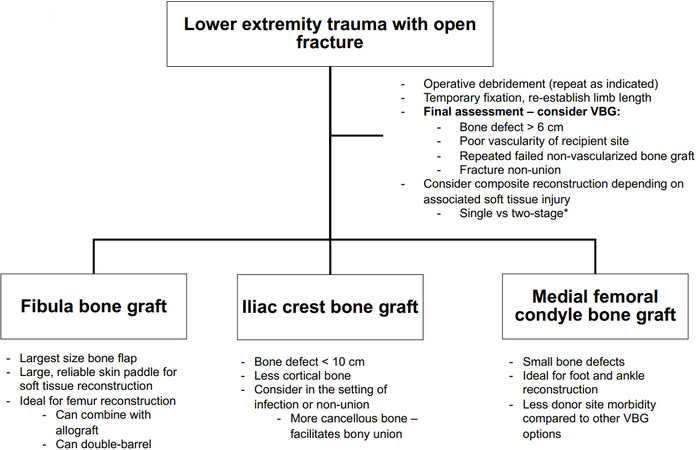
The three primary donor site options for vascularized bone grafting for the lower extremity include the fibula, iliac crest, and medial femoral condyle [Figure 1]. Historically, vascularized rib grafts have been described; however, they have fallen out of favor. Flap selection is determined predominantly based upon the size and location of the bony defect, flap availability, donor site morbidity, and flap success rates. General considerations when selecting the ideal vascularized bone graft for a post-traumatic bone defect in the lower extremity are outlined in Figure 2.
Figure 1. Characteristics of vascularized bone graft options for traumatic lower extremity bone defect reconstruction.
Figure 2. Selecting a vascularized bone graft for lower extremity traumatic bone defects in adults. *The optimal timing for soft tissue reconstruction and vascularized bone grafting should be determined on a case-by-case basis, keeping in mind the limitations of each approach. VBG: Vascularized bone graft.
The fibula flap is the most commonly used vascularized bone graft for extremity reconstruction. It provides a strong cortical strut that is straight, and therefore ideal for extremity reconstruction. The vascularized fibula flap can be used to reconstruct defects up to 26 cm in length[18]. The flap pedicle is the peroneal artery with associated peroneal veins. The composition of this flap can vary to include skin, fascia and muscle as indicated by the defect requirements. A skin paddle up to 20 by 10 cm can be reliably transferred and used for flap monitoring. Additionally, this bone flap has an endosteal and periosteal blood supply, which allows for the option to create a double-barrel construct with a single pedicle using a transverse osteotomy in the diaphysis of the graft for femur or tibial reconstruction[18]. For large intercalary defects of the femur, the vascularized fibular graft can be combined with a massive cortical allograft (Capanna technique). The allograft provides immediate strength to the construct, while the fibula graft provides the previously noted biologic benefits of vascularized bone graft (potential for osteogenesis, ability to hypertrophy, and faster time to the union)[12].
Vascularized iliac crest bone grafts have been described for lower extremity bone reconstruction. This corticocancellous bone has a slight curve, which is undesirable in long bone reconstruction. This maximal graft length from this donor site is only 10 to 12 cm. The flap pedicle is the deep circumflex iliac vessel. An associated skin paddle based on the deep circumflex iliac vessels alone is reportedly unreliable - if a large skin paddle is required for osteocutaneous reconstruction, a double anastomosis including the superficial circumflex iliac vessels should be performed[14,18-20].
The medial femoral condyle (MFC) flap has been popularized for use in the setting of post-traumatic avascular necrosis of the talus and navicular bones and the setting of foot and ankle fracture nonunions. The MFC is typically a thin corticoperiosteal graft that includes periosteum and outer cortical bone, with or without underlying cancellous bone. The dominant flap pedicle is the descending genicular artery; however, the flap can be supported by the medial superior geniculate artery (though this results in a shorter pedicle length). The advantage of this flap is its thin, pliable nature which allows for shaping to fit small, irregular bone defects[9]. When used for structural support, the maximal graft length is 3 cm in length; however, flaps up to 5 cm × 7 cm have been described[21,22]. The MFC can be raised with an associated skin paddle based on the saphenous artery branch of the descending geniculate artery pedicle (medial knee, up to 70 cm2) or a more distal descending geniculate artery cutaneous perforator (medial distal thigh and proximal leg, up to 361 cm2)[23,24]. Because of variable branching patterns and available distal cutaneous perforators, the size of the skin paddle associated with an MFC flap is inconsistent[24,25].
PRE-OPERATIVE CONSIDERATIONS
In the post-traumatic setting, multiple operative debridements are often required to adequately address contamination and removal of devitalized bone and soft tissues with the goal of eradicating potential nidi for infection. Provisional bony stabilization is typically obtained with external fixation. In the setting of associated soft tissue loss (Gustillo IIIB or IIIC), some authors recommend single-stage reconstruction with composite bone grafting, while others advocate for soft tissue reconstruction followed by vascularized bone grafting in a second stage 6-8 weeks later. The advantages of one-stage reconstruction include reduction of overall healing time and simultaneous soft tissue and bone reconstruction, with the theoretical disadvantage of increased risk of infection and loss of the vascularized bone graft. The disadvantages associated with staged reconstruction include increased scar burden and limited availability of recipient vessels for subsequent vascularized bone grafting[26-31]. The optimal timing for soft tissue reconstruction and vascularized bone grafting should be determined on a case-by-case basis, keeping in mind the limitations of each approach.
Evaluation of the donor and recipient vascular anatomy in the setting of lower extremity trauma is an important component of the pre-operative workup. This includes clinical evaluation and Doppler ultrasound examination. Angiography of the recipient and/or donor sites can be performed if there are abnormalities on exam and for pre-operative planning. In the setting of high-energy trauma, the extent of injury to the potential recipient vessels can be assessed with angiography[18,20]. However, pre-operative angiography may not reliably delineate the extent of vascular injury in the recipient vessels following trauma or in patients with chronic infections. Vein grafts may be required based on the availability of healthy recipient vessels outside of the zone of injury[13].
Patient age is also an important consideration. Traumatic bone defects in the pediatric population are more rare and have better prognoses compared to the adult population. The integrity of their relatively thick, vascular, and cellularly active periosteum can be used to guide the reconstruction of pediatric traumatic bone defects. If the periosteum is intact, bone reconstruction may not be necessary with appropriate stabilization. A non-vascularized autograft may be used for bone defects with partially intact periosteum. In patients with no periosteum or with nonunion due to infection, bony reconstruction using the induced membrane technique followed by autograft bone, bone transport, or vascularized bone grafting has been described. Case series describing outcomes following reconstruction of pediatric bone defects in the setting of trauma are limited[32]. However, the use of vascularized bone grafts in the pediatric population has been shown to be a reliable reconstructive option following tumor resections of the extremities with flap survival rates of over 92%-92% for fibula, 100% for iliac crest, and 96% for MFC flaps[33-36]. Long-term outcomes following vascularized fibula flaps for bone sarcoma reconstruction in pediatric patients were evaluated by Ruiz-Moya et al.[37] and Ghoneimy et al.[38]. In these series (68 patients total), the rate of graft fracture was 33.3% to 34%, and the rate of non-union was 11% to 13.8%[37,38].
DEFECT RECONSTRUCTION
Following vascularized bone graft harvest and recipient site preparation, the bone graft is inset to bridge the bony defect, and the flap is revascularized. Several options have been described in the literature for graft stabilization including plates and screws, screws only, k-wire fixation, intramedullary nail, press-fit (MFC flaps), and external fixation[3,18,25,39,40]. Stability can also be augmented with graft inset into the medullary canal of the recipient bone[18]. Many authors advocate for ancillary cancellous bone graft placement at the junction between the graft and the recipient bone has been to accelerate healing[18,40]. Han et al.[40] compared vascularized bone graft fixation techniques (stable internal fixation vs. unstable internal fixation vs. external fixation). They reported significantly higher rates of primary bone union with stable internal fixation ± ancillary bone grafting (71%) compared to external fixation ± ancillary bone grafting (47%). The type of skeletal fixation with or without ancillary bone grafting had no significant effect on overall bone union rates[40]. Many authors describe a preference for stabilization with Ilizarov frames - the reported advantages include the ability to adjust bone compression/distraction, early weight-bearing, and decreased risk of infection with an external device[3,41]. The use of large plates for fixation can provide too rigid fixation , which can result in a stress-shielding effect on the bone graft[18,42].
OUTCOMES
Bone union
Osseous union is an indicator of bone healing. This can be assessed using plain film radiographs to evaluate for the presence of a bridging callus or absence of the fracture line. Reported rates of primary union for vascularized bone grafting in the post-traumatic setting range from 79% to 88.5% (Minami et al.[43], 26 of 33 flaps; Yazar et al.[39], 54 of 61 flaps)[39,43]. Han et al.[40] evaluated vascularized bone grafting with the fibula and iliac crest flaps to all skeletal defects (mandible, clavicle, upper extremity, pelvis, lower extremity). They reported significantly lower rates of primary and overall union in patients with osteomyelitis - 46% (29 of 60 flaps) rate of primary union, 76% (46 of 60 flaps) rate of overall union[40]. In a systematic review by
Several authors have attempted to compare primary union rates between vascularized bone flaps.
The time to union of vascularized bone grafts in the setting of lower extremity trauma has been evaluated by Yazar et al.[39] and Pelissier et al.[13] in single center retrospective reviews. It is reported as an average time to union for all included vascularized bone grafts (regardless of donor site). Yazar et al.[39] evaluated one-stage composite bone and soft tissue reconstruction in the setting of lower extremity trauma using fibula (50), iliac crest (6) and rib (5) vascularized bone grafts. The average time to primary union in this series of 61 patients was 6.9 months, with an average time to overall union of 8.5 months[39]. Pelissier et al.[13] reported a series of 24 patients who underwent vascularized bone grafting (10 iliac crest, 12 fibula, 2 lateral arm flaps), with an average time to primary union of 11.5 months. Another single center review of 14 patients who underwent vascularized fibula flap for post-traumatic lower extremity reconstruction reported an average time to union of 3.9 months[45]. The average time to union following MFC flaps for all lower extremity reconstruction is 3.5 months in a systematic review of the literature by Kazmers et al.[44]. However, the average time to union in the setting of foot and ankle reconstruction with MFC flap was reported to be 7.1 months in a retrospective review of 30 MFC flaps by Stranix et al.[25].
Flap failure
The reported rate of flap failure, defined as unsalvageable vascular thrombosis resulting in total flap loss, in the setting of post-traumatic bone defect ranges from 3.1% to 10% (Yazar et al.[39], 2 of 63 flaps; Lin et al.[14], 3 of 68 flaps; Hierner and Wood[19], 1 of 10 flaps). Higher rates of flap failure following reconstruction for chronic osteomyelitis compared to post-traumatic bone defects have been reported - 20.7% (Lin et al.[14], 6 of 29 flaps) to 25% flap failure rate (Hierner and Wood[19], 6 of 24 flaps)[39].
The rate of flap failure based on donor site was assessed by Lin et al.[14]. Ninety-seven vascularized bone transfers to the lower extremity from 1991 to 1995 were included for retrospective review. The indications for bone reconstruction were post-debridement of osteomyelitis and post-traumatic bone defects. The flap failure rates in this series were 4.7% following fibula flaps, 13.6% following rib flaps, and 27.3% following iliac bone flaps. Stranix et al.[25] reported no total flap losses in their 30 patient series of MFC flaps for foot and ankle reconstruction.
Stress fracture
Stress or fatigue fractures of the vascularized bone graft can occur, especially in lower extremity reconstruction. Following vascularized bone graft reconstruction, excessive mechanical loading before the graft has had an opportunity to adequately hypertrophy can result in fracture. The rates of stress fracture following vascularized fibula grafts for post-traumatic bone defects ranges from 11.5% to 13.1%[14,26,39,43].
Donor site morbidity
Donor site morbidity profiles can have a significant impact on the surgical decision-making process. Limited donor site morbidity has been described following MFC flaps when compared to vascularized free fibula flap and iliac crest bone graft. Multiple systematic reviews have been performed to elucidate the true incidence of complications associated with these vascularized bone graft donor sites. The donor site morbidities based on vascularized bone graft are outlined in Table 1.
Donor site morbidity following vascularized bone graft for lower extremity traumatic bone defects
| Fibula | Iliac crest | MFC |
| EARLY COMPLICATIONS | ||
| Infection dehiscence necrosis Delayed wound healing Skin graft loss Compartment syndrome | Infection Dehiscence hernia | Altered sensation Hematoma seroma Delayed wound healing |
| LATE COMPLICATIONS | ||
| Decreased ankle range of motion Altered sensation Persistent pain Claw toe Ankle instability Reduced muscle strength Gait abnormality Reduced muscle strength | Altered sensation Persistent pain Gait disturbance | Persistent knee pain Femur fracture |
Ling et al.[46] performed a systematic review of the literature and calculated weighted mean incidences for early and late donor site complications following free fibula flap. Early complications included infection (1.1%), dehiscence (7.0%), necrosis (7.3%), delayed wound healing (17.4%) and skin graft loss (8.1% partial graft loss, 4.7% complete graft loss). Of note, the incidence of wound healing complications was higher in donor sites that were skin grafted compared to those that were closed primarily (19.0% vs. 9.9%). The most common late complication was a limited range of motion of the ankle (11.5%) followed by altered sensation (7.0%), persistent pain (6.5%), claw toe (6.1%), ankle instability (5.8%), reduced muscle strength (4.0%), gait abnormality (3.9%), and dorsiflexion of the great toe (3.6%)[46]. Compartment syndrome of the lower leg is rare, however severe, complication following vascularized free fibula flap - a rate of 3% was reported in a recent meta-analysis by Gu et al.[47] in the setting of mandibular reconstruction.
Early and long-term donor site morbidities following vascularized iliac bone flap for mandibular reconstruction were evaluated by Gu et al.[47] in a 2021 systematic review and meta-analysis. The most common donor site morbidities were late complications - chronic altered sensation (43%), pain (26%), and gait disturbance (20%)[47]. The altered sensation is likely related to injury to the lateral femoral cutaneous nerve with resulting lateral thigh numbness/tingling. The reported early donor site morbidities (wound infection, dehiscence, and hernia) ranged from 3% to 16%[47]. The iatrogenic hernia is a rarely reported complication (3% in the previously cited meta-analysis) and is thought by some authors to correlate with the size of the flap[47,48]. Donor site morbidity has been compared in the craniomaxillofacial literature between free fibula flaps and vascularized iliac bone grafts. The fibula group had less immediate post-operative pain and returned to unaided walking faster, while patients who underwent iliac bone graft had a higher incidence of altered sensation and gait abnormality[49].
A systematic review of the literature evaluating donor site morbidity following vascularized bone grafts from the distal femur by Giladi et al.[50] found a low rate of donor site complications. The most commonly reported donor site morbidities included persistent knee pain (which commonly lasts up to 3 months), and temporary altered sensation (parethesias or sensitivity) - < 2% of patients in the included studies had permanent altered sensation. The authors suggest that sensory changes could be related to saphenous nerve injury associated with osteocutaneous flap harvest. There are no reports in the literature of post-operative knee instability or limitations to knee range of motion. Secondary procedures were performed in 2.5% of patients included for review (6 flaps); procedures included: hematoma evacuation, seroma evacuation, wound debridement, and open reduction and internal fixation of femur fracture[50]. Post-operative femur fracture has been reported in two patients in the literature[22].
CONCLUSIONS
Vascularized bone grafts may be required for the reconstruction of bone defects in the setting of lower extremity trauma. Indications include bony defect greater than 6 cm, fracture non-union, and previously failed reconstruction with non-vascularized bone grafts[12-14]. Single-stage and two-stage approaches have been described in the literature - immediate, often composite, free flap reconstruction vs. immediate soft tissue coverage followed by second stage bony reconstruction[26-31]. Free fibula flaps provide the largest size bone flap, ideal for larger bone defects and femur defects that would benefit from double-barreled reconstruction[18]. Vascularized iliac bone flaps provide less cortical bone for reconstruction, and are therefore ideal for defects that are less than 10 cm in length. However, it can be harvested with associated cancellous bone which can facilitate bony union[14,18-20]. MFC flaps are ideal for smaller bone defects, and can be implemented in the setting of foot and ankle trauma[25].
DECLARATIONS
Authors’ contributionsMade substantial contributions to conception and design of the paper and provided mentorship and revisions on the manuscript: Stranix JT
Performed literature review, composition of manuscript: Schaeffer CV
Availability of data and materialsNot applicable.
Financial support and sponsorshipNone.
Conflicts of interestBoth authors declared that there are no conflicts of interest.
Ethical approval and consent to participateNot applicable.
Consent for publicationNot applicable.
Copyright© The Author(s) 2022.
REFERENCES
2. DeLong WG, Born CT, Wei SY, Petrik ME, Ponzio R, Schwab CW. Aggressive treatment of 119 open fracture wounds. J Trauma 1999;46:1049-54.
3. Battiston B, Santoro D, Baido RL, Pasquero F. Treatment of acute bone defects in severe lower limb Trauma. Injury 2019;50 Suppl 5:S40-5.
4. Taylor GI, Miller GD, Ham FJ. The free vascularized bone graft. A clinical extension of microvascular techniques. Plast Reconstr Surg 1975;55:533-44.
5. Buncke HJ, Furnas DW, Gordon L, Achauer BM. Free osteocutaneous flap from a rib to the tibia. Plast Reconstr Surg 1977;59:799-804.
6. Taylor GI, Watson N. One-stage repair of compound leg defects with free, revascularized flaps of groin skin and iliac bone. Plast Reconstr Surg 1978;61:494-506.
7. Doi K, Sakai K. Vascularized periosteal bone graft from the supracondylar region of the femur. Microsurgery 1994;15:305-15.
8. Sparks DS, Wagels M, Taylor GI. Bone reconstruction: a history of vascularized bone transfer. Microsurgery 2018;38:7-13.
9. Doi K, Hattori Y. Vascularized bone graft from the supracondylar region of the femur. Microsurgery 2009;29:379-84.
10. Gustilo RB, Anderson JT. Prevention of infection in the treatment of one thousand and twenty-five open fractures of long bones: retrospective and prospective analyses. J Bone Joint Surg Am 1976;58:453-8.
11. Winquist RA, Hansen ST Jr, Clawson DK. Closed intramedullary nailing of femoral fractures. A report of five hundred and twenty cases. J Bone Joint Surg Am 1984;66:529-39.
12. Houben RH, Rots M, van den Heuvel SCM, Winters HAH. Combined massive allograft and intramedullary vascularized fibula as the primary reconstruction method for segmental bone loss in the lower extremity: a systematic review and meta-analysis. JBJS Rev 2019;7:e2.
13. Pelissier P, Boireau P, Martin D, Baudet J. Bone reconstruction of the lower extremity: complications and outcomes. Plast Reconstr Surg 2003;111:2223-9.
14. Lin CH, Wei FC, Chen HC, Chuang DC. Outcome comparison in traumatic lower-extremity reconstruction by using various composite vascularized bone transplantation. Plast Reconstr Surg 1999;104:984-92.
15. Goldberg VM, Shaffer JW, Field G, Davy DT. Biology of vascularized bone grafts. Orthop Clin North Am 1987;18:197-205.
16. Cutting CB, McCarthy JG. Comparison of residual osseous mass between vascularized and nonvascularized onlay bone transfers. Plast Reconstr Surg 1983;72:672-5.
17. Shi LL, Garg R, Jawa A, et al. Bony hypertrophy in vascularized fibular grafts. Hand (N Y) 2022;17:106-13.
18. Malizos KN, Zalavras CG, Soucacos PN, Beris AE, Urbaniak JR. Free vascularized fibular grafts for reconstruction of skeletal defects. J Am Acad Orthop Surg 2004;12:360-9.
19. Hierner R, Wood MB. Comparison of vascularised iliac crest and vascularised fibula transfer for reconstruction of segmental and partial bone defects in long bones of the lower extremity. Microsurgery 1995;16:818-26.
20. Nusbickel FR, Dell PC, McAndrew MP, Moore MM. Vascularized autografts for reconstruction of skeletal defects following lower extremity trauma. A review. Clin Orthop Relat Res 1989;243:65-70.
21. Bishop A. Vascularized bone grafting. In: Green DP, Hotchkiss RN, Pederson WC WS, editors. Green’s operative hand surgery. New York: Churchilll Livingstone; 2005. p. 1777-811.
22. Haddock NT, Alosh H, Easley ME, Levin LS, Wapner KL. Applications of the medial femoral condyle free flap for foot and ankle reconstruction. Foot Ankle Int 2013;34:1395-402.
23. Iorio ML, Masden DL, Higgins JP. Cutaneous angiosome territory of the medial femoral condyle osteocutaneous flap. J Hand Surg Am 2012;37:1033-41.
24. Rysz M, Grabczan W, Mazurek MJ, Krajewski R, Grzelecki D, Ciszek B. Vasculature of a medial femoral condyle free flap in intact and osteotomized flaps. Plast Reconstr Surg 2017;139:992-7.
25. Stranix JT, Piper ML, Azoury SC, et al. Medial femoral condyle free flap reconstruction of complex foot and ankle pathology. Foot Ankle Orthop 2019;4:2473011419884269.
26. Beris AE, Lykissas MG, Korompilias AV, et al. Vascularized fibula transfer for lower limb reconstruction. Microsurgery 2011;31:205-11.
27. Cierny G. Primary versus delayed soft tissue coverage for severe open tibial fractures. A comparison of results. Clin Orthop Relat Res 1983:54-63.
28. Lin CH, Wei FC, Levin LS, et al. Free composite serratus anterior and rib flaps for tibial composite bone and soft-tissue defect. Plast Reconstr Surg 1977;99:1656-65.
29. Doi K, Kawakami F, Hiura Y, Oda T, Sakai K, Kawai S. One-stage treatment of infected bone defects of the tibia with skin loss by free vascularized osteocutaneous grafts. Microsurgery 1995;16:704-12.
30. Yaremchuk MJ, Brumback RJ, Manson PN, Burgess AR, Poka A, Weiland AJ. Acute and definitive management of traumatic osteocutaneous defects of the lower extremity. Plast Reconstr Surg 1987;80:1-14.
31. Malizos KN, Nunley JA, Goldner RD, Urbaniak JR, Harrelson JM. Free vascularized fibula in traumatic long bone defects and in limb salvaging following tumor resection: comparative study. Microsurgery 1993;14:368-74.
32. de Gauzy J, Fitoussi F, Jouve JL, Karger C, Badina A, Masquelet AC; French Society of Orthopaedic Surgery and Traumatology (SoFCOT). Traumatic diaphyseal bone defects in children. Orthop Traumatol Surg Res 2012;98:220-6.
33. El-Gammal TA, El-Sayed A, Kotb MM. Reconstruction of lower limb bone defects after sarcoma resection in children and adolescents using free vascularized fibular transfer. J Pediatr Orthop B 2003;12:233-43.
34. Cashin M, Coombs C, Torode I. A-frame free vascularized fibular graft and femoral lengthening for osteosarcoma pediatric patients. J Pediatr Orthop 2018;38:e83-90.
35. Gorski SM, Dong C, Krieg AH, Haug M. Vascularized bone graft reconstruction following bone tumor resection at a multidisciplinary sarcoma center: outcome analysis. Anticancer Res 2021;41:5015-23.
36. Houdek MT, Rose PS, Milbrandt TA, Stans AA, Moran SL, Sim FH. Comparison of pediatric intercalary allograft reconstructions with and without a free vascularized fibula. Plast Reconstr Surg 2018;142:1065-71.
37. Ruiz-Moya A, Lagares-Borrego A, Sicilia-Castro D, et al. Pediatric extremity bone sarcoma reconstruction with the vascularized fibula flap: observational study assessing long-term functional outcomes, complications, and survival. J Plast Reconstr Aesthet Surg 2019;72:1887-99.
38. Ghoneimy AME, Sherbiny ME, Kamal N. Use of vascularized fibular free flap in the reconstruction of the femur in pediatric and adolescent bone sarcomas: complications and functional outcome. J Reconstr Microsurg 2019;35:156-62.
39. Yazar S, Lin CH, Wei FC. One-stage reconstruction of composite bone and soft-tissue defects in traumatic lower extremities. Plast Reconstr Surg 2004;114:1457-66.
40. Han CS, Wood MB, Bishop AT, Cooney WP 3rd. Vascularized bone transfer. J Bone Joint Surg Am 1992;74:1441-9.
42. Levin LS. Vascularized fibula graft for the traumatically induced long-bone defect. J Am Acad Orthop Surg 2006;14:S175-6.
43. Minami A, Kasashima T, Iwasaki N, Kato H, Kaneda K. Vascularised fibular grafts. An experience of 102 patients. J Bone Joint Surg Br 2000;82:1022-5.
44. Kazmers NH, Thibaudeau S, Steinberger Z, Scott Levin L. Upper and lower extremity reconstructive applications utilizing free flaps from the medial genicular arterial system: a systematic review. Microsurgery 2018;38:328-43.
45. Ael-S, Badawy E, Hasan M, El-Nakeeb RM. Management of post-traumatic bone defects of the tibia using vascularised fibular graft combined with Ilizarov external fixator. Injury 2016;47:969-75.
46. Ling XF, Peng X. What is the price to pay for a free fibula flap? Plast Reconstr Surg 2012;129:657-74.
47. Gu Y, Ma H, Shujaat S, et al. Donor- and recipient-site morbidity of vascularized fibular and iliac flaps for mandibular reconstruction: a systematic review and meta-analysis. J Plast Reconstr Aesthet Surg 2021;74:1470-9.
48. Yilmaz M, Vayvada H, Menderes A, Demirdover C, Kizilkaya A. A comparison of vascularized fibular flap and iliac crest flap for mandibular reconstruction. J Craniofac Surg 2008;19:227-34.
49. Ling XF, Peng X, Samman N. Donor-site morbidity of free fibula and DCIA flaps. J Oral Maxillofac Surg 2013;71:1604-12.
Cite This Article
Export citation file: BibTeX | RIS
OAE Style
Schaeffer CV, Stranix JT. Tackling bone loss of the lower extremity: vascularized bone grafting. Plast Aesthet Res 2022;9:27. http://dx.doi.org/10.20517/2347-9264.2021.122
AMA Style
Schaeffer CV, Stranix JT. Tackling bone loss of the lower extremity: vascularized bone grafting. Plastic and Aesthetic Research. 2022; 9: 27. http://dx.doi.org/10.20517/2347-9264.2021.122
Chicago/Turabian Style
Schaeffer, Christine V., John T. Stranix. 2022. "Tackling bone loss of the lower extremity: vascularized bone grafting" Plastic and Aesthetic Research. 9: 27. http://dx.doi.org/10.20517/2347-9264.2021.122
ACS Style
Schaeffer, CV.; Stranix JT. Tackling bone loss of the lower extremity: vascularized bone grafting. Plast. Aesthet. Res. 2022, 9, 27. http://dx.doi.org/10.20517/2347-9264.2021.122
About This Article
Special Issue
Copyright
Data & Comments
Data
 Cite This Article 12 clicks
Cite This Article 12 clicks











Comments
Comments must be written in English. Spam, offensive content, impersonation, and private information will not be permitted. If any comment is reported and identified as inappropriate content by OAE staff, the comment will be removed without notice. If you have any queries or need any help, please contact us at support@oaepublish.com.