Skin collagen through the lifestages: importance for skin health and beauty
Abstract
Collagen-based supplements have become a keystone in the management of the ageing process, with proven ability to repair skin damage, bestowing a youthful and healthy appearance sought in the pursuit of beauty. Collagen is an essential scaffold protein that gives smoothness and elasticity to skin, but its production declines with age. Finding ways to tackle this problem is now strongly promoted as an effective way to transform skin and hair, repairing age-related deterioration. A growing number of scientific studies show exciting evidence that it is possible to rejuvenate ageing or damaged skin, improve function of worn joints, and support personal wellbeing and vitality. In recent times, research on the mechanisms which impact the production of collagen in skin and the ideal organization into functional fibres which give skin its characteristic elasticity and firmness has provided new insights into how this bio-scaffold can support cells, tissues and organs. The factors which influence collagen production over a lifetime (e.g., puberty, pregnancy, menopause, andropause), intrinsic factors (e.g., genetics, age, ethnicity) and extrinsic factors (e.g., UV-radiation, pollution, smoking) and the potential for new technologies, ingredients and devices to restore collagen and matrix components to their optimal condition are improving the ability to deliver anti-aging strategies with unprecedented results. This paper will review skin collagen production, structure and function throughout the lifestages, emphasizing its relationship with health, appearance and beauty.
Keywords
Introduction
Synthesis and structure of collagen in skin
Collagen Synthesis by fibroblasts
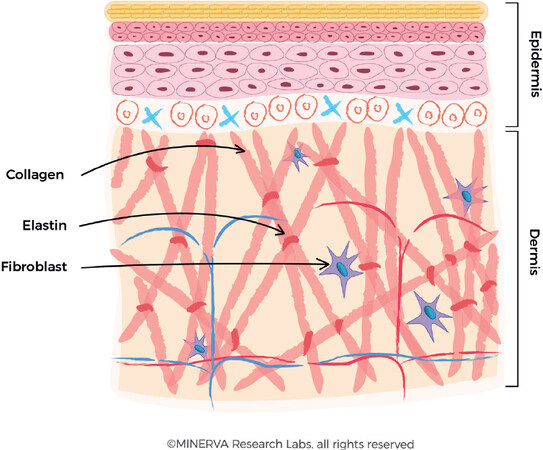
Collagen provides the support matrix/mattress underpinning healthy skin and is a key determinant to the preservation of skin firmness and elasticity[1,2]. Type I is the main collagen found in skin, representing 80%-90% of skin collagen. It is produced by cells called fibroblasts, which are a mesenchymal cell type, found predominantly in the dermis[3]. Fibroblasts also produce elastin protein which gives skin the flexibility to stretch by facilitating a long-range deformability, followed by a recoil to allow tissues to return to their original conformation[4,5]. This is a critical functionality to maintain the skin elasticity and resilience [Figure 1]. Another product of fibroblast metabolic function is the production of glycosaminoglycans (GAGs) which are long unbranched heteropolysaccharides such as hyaluronates and dermatan sulphate (the most abundant GAG in skin). The unique combination of high viscosity, high hygroscopicity and low compressibility are key to their many functions, including maintenance of the skin’s essential moisture content[6].
Figure 1. The production of collagen fibres in the dermis. The fibroblast secretes the procollagen fibre into the extracellular matrix, where they form larger collagen bundles. Elastin is also secreted and assembled into the collagen-based macromolecular structure. (By permission of MINERVA Research Labs Ltd - London)
Fibroblasts are sensitive to the physical tension of the extracellular matrix (ECM) in which they are embedded, and to biochemical stimuli and signalling pathways, both of which can induce fibroblast activation and proliferation[7]. Small molecular weight, diffusible ligands can bind to receptors located on the fibroblast extracellular membrane inducing their activation. Physical tension in the ECM can directly cause activation of mechanoreceptors and anchoring fibrils of the inherent cytoskeletal framework and initiate signalling pathways involved in cell-to-ECM communication[8]. The activation of fibroblasts results in an increase in the production of collagen, elastin and associated GAGs[9].
Many anti-ageing strategies are targeted at influencing production of ECM components by fibroblasts. A wide range of ligands can influence fibroblast proliferation and activation, including bioactive peptides, antioxidants, retinoids, vitamins, ω6- and ω3-fatty acids, growth factors, hydroxy acids and a bewildering array of botanical extracts[10-12]. A common theme for the majority of these ingredients is that they can influence, either directly or indirectly, the production of collagen and ECM components.
From early adulthood, fibroblasts become less active and collagen production declines by about 1.0%-1.5% a year[13,14]. This can also be aggravated by certain lifestyle choices like smoking and external factors like sun exposure[15]. Ongoing sunlight and pollution exposure and reduced efficiency in eliminating free radical chemicals add to the damage. Many studies have shown that if collagen peptides (and other active compounds) are ingested they will travel throughout the body, including to sites where fibroblasts are present. This stimulates fibroblasts to produce more collagen, elastin and hyaluronic acid, thereby rejuvenating skin and other tissues. This mechanism is key to the successful production of collagen reported in clinical studies following long term supplement use and the consequent reported improvement in skin elasticity and hydration.
A recent in vitro study from Edgar et al.[16] has shown that hydrolysed collagen peptides significantly increase collagen and elastin synthesis by fibroblasts while significantly inhibiting the release of two collagenases, namely metalloproteinase-1 (MMP-1) and MMP-3. The research primarily investigated the interactions between collagen peptides and other constituents (including GAGs and antioxidants) present in the hydrolysed collagen-based nutraceutical, Gold Collagen® Forte, on normal primary dermal fibroblast function. The effects of the addition of collagen peptides, alone or in combination with other bioactive and antioxidant constituents, were tested and compared to the effect of media alone. The increase in collagen and elastin synthesis was accompanied by a decrease in the activity of MMP enzymes. MMP enzymes are responsible for matrix breakdown and elastin degradation and an increase in MMP activity is associated with UV-irradiation and reactive oxygen based free radical damage to the ECM components[17,18]. The data provided a scientific, cell-based, rationale for the positive effects of collagen-based nutraceutical supplements on skin properties, suggesting that enhanced formation of stable dermal fibroblast-derived ECM may follow their oral consumption.
Collagen fibril formation and characterization
There are about 28 different forms of collagen expressed in the body, of which the molecular biology, biochemistry and ECM structural and architectural components have been reviewed in detail by Shoulders et al.[2,19] and in concise overview by Kadler et al.[20]. The family of proteins includes both fibril-forming and non-fibril-forming proteins, however the main collagens involved in skin architecture and physiology are the fibril-forming types, predominantly Type I and Type III (the Roman Numerals denote the order of discovery). Each protein is encoded by a series of genes, with gene loci for the members of the collagen family labelled with the abbreviation “COL”, followed by annotation for both collagen type and constituent chains, e.g., COL1A1 for the α1 chain of Type I collagen[21]. Type I collagen, the prototype and also the most abundant member, has a long chain triple helix structure, comprising a heterotrimer of two identical αl(l) chains and one α2(I) chain [Figure 2]. A major structural determinant of the protein is a triple-helical structure of three polypeptide chains with a characteristic amino acid sequence (Gly-X-Y) which is repeated frequently across the fibril structure, where Gly is glycine and X and Y would frequently be amino acids such as proline and hydroxyproline[22].
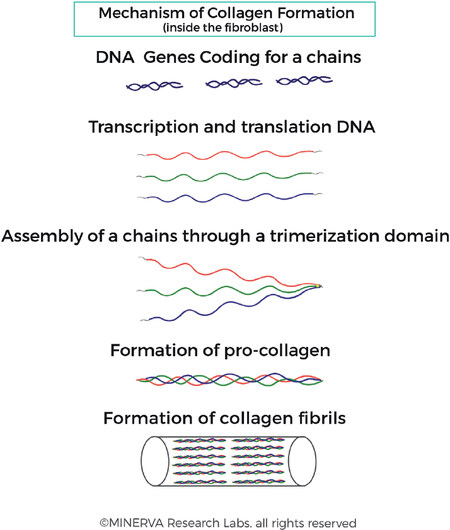
Figure 2. Collagen fibril formation. Collagen genes are transcribed into RNA and translated into protein in the fibroblast cell. Post-translational processing occurs, followed by binding of the 3 individual chains at the C-terminus. The 3 chains are tightly bound together and supported with cross-links which stabilise the structure. This trimerization process allows assembly of α-chains which are further assembled into fibrils. (By permission of MINERVA Research Labs Ltd - London)
Genes coding for the alpha chains of collagens are transcribed into RNA and translated into protein in the endoplasmic reticulum of the fibroblast cell and processed within a secretory vesicle. Binding of the 3 individual chains at the C-terminus initiates formation of the triple helix, which proceeds towards the N-terminus in a zipper-like manner [Figure 2]. This allows assembly of α-chains through a trimerization process to form pro-collagens, which are further assembled into fibrils[23]. Following secretion of the collagen triple helix structure into the ECM, post-translational cleavage of the N- and C-terminal peptides occurs [Figure 3].
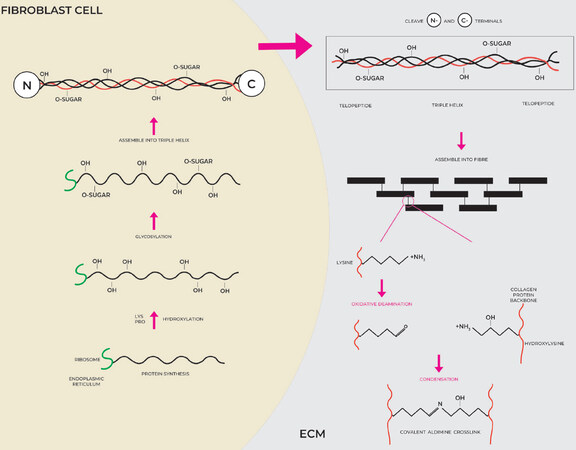
Figure 3. Collagen synthesis, secretion into the ECM and cross-linking. Collagens are translated into protein on the ribosomes of the endoplasmic reticulum inside the fibroblast cell. Hydroxylation and glycosylation occur before the 3 helical strands are woven together to form the procollagen species. After secretion into the ECM, the N-terminal and C-terminal ends are cleaved and the tropocollagen units can be assembled into larger structure, which are held together via crosslinked residues between the Lysine aldehyde derivative of one collagen strand and the corresponding hydroxylysine of the opposite strand. (By permission of MINERVA Research Labs Ltd - London)
The uniquely high content of the amino acids proline (or more specifically imino acid, wherein the secondary amine results in a rotationally constrained rigid-ring structure which imparts unique structural stability) and lysine allows a range of post-translational modifications due to the hydroxylation of proline and lysine residues[24]. Lysine hydroxylation allows for crosslinking of intertwined fibres and gives the insoluble protein unique characteristics including thermal stability, mechanical strength and a 3-dimensional structure amenable to production of coiled fibres which are very resilient to the varied mechanical and biological forces experienced during a lifetime [Figure 3][25].
Type I collagen is present in skin, tendon, vasculature, organs and bone (it is the main component of the organic part of the bone, a scaffold which is subsequently mineralized to produce a structure stronger than steel and yet light enough to facilitate mobility and speed). Type II is predominantly present in cartilage, a substance many times smoother than glass, with a very low friction coefficient, yet it is not brittle and does not crack under pressure. Type III is commonly found alongside Type I and usually represents about 15% of skin collagen. It is a homotrimer composed of three identical α1 peptide chains.
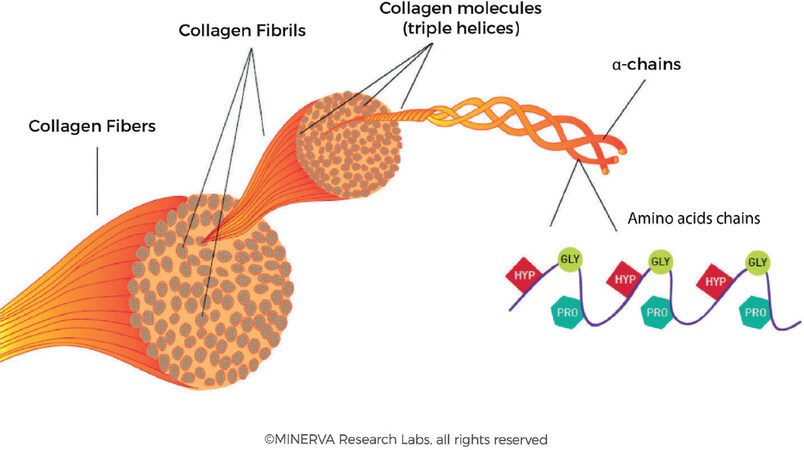
Collagen fibres form extensive and robust networks providing the dermis with strength, firmness and elasticity. As shown in Figure 4, a collagen fibre is typically up to 3 μm in diameter and has a characteristic coiled structure[2,26]. A collagen fibre is essentially comprised of bundles of smaller fibrils. Collagen fibrils are approximately 10 to 300 nm in diameter and several micrometres in length. A collagen fibril is a bundle of triple stranded collagen molecules (about 1.5 nm in diameter and approximately 300 nm long). This triple helix, coiled structure is stereo-dynamically favourable to allow strands to be interwoven together and this incredibly robust structure can persist in tissues for many years[27,28].
Figure 4. The organization of collagen fibrils into fibre bundles. Individual α-chains are woven into triple helices via a zipper mechanism. Bundles of triple helices form fibrils and these fibrils are aggregated into larger fibres. (By permission of MINERVA Research Labs Ltd - London)
Formation of fibres is dependent on interaction with other ECM components including elastin proteins and GAGs. All GAGs except hyaluronan (HA) bind to collagen via electrostatic interaction under normal physiological conditions[29]. The hypothesis is that proteoglycan-collagen interaction directly influences the deposition of collagen fibres in situ, although further research is required to clarify the mechanisms involved. Protein and GAG interactions thus determine the synthesis, secretion and formation of collagen-based matrix, whereas the osmotic equilibrium in connective tissue is determined by the rapid turnover of GAGs such as HA and dermatan sulphate[9,30].
Non-invasive imaging systems can be used to visualise and quantify collagen in the skin. Ultrasound devices are available for use in clinical studies depending on the required application and study design, e.g., measuring collagen in skin versus tendon, body site tested (arm versus face), resolution (µm), sensitivity and depth of skin measurement (papillary versus reticular dermis). The range of frequencies for skin imaging is recommended to be 20-25 Mhz. Imaging by confocal microscopy has gained immense importance as technological advances in resolution have enabled dermal components to be visualized and quantified in exquisitely fine detail. Multiphoton microscopy (MPM) and reflectance confocal microscopy (RCM) have demonstrated promising results in imaging skin micromorphology. The RCM has become a vital tool in analysing accurately the cellular images of in vivo human skin to study the variations of cellular parameters such as cell size, nucleus size, keratinocyte morphology (which becomes increasingly irregular with age or inflammation) and in diagnosing morphometric features of collagen fibre type[31]. In the dermis fibrillary grading of collagen types, classified as hypo-reflective versus hyper-reflective structures indicating fibre intensity and definition, whereby a hypo-reflective collagen makes it difficult to identify single fibres, compared to a hyper-reflective collagen fibre which is well defined and fibrous in nature[32]. Further characterisation of the type of collagen as thin reticulated, coarse, huddled, or curdled, allows more detailed description of skin collagen and changes observed throughout the lifestages.
In an elegant series of studies, Ueda et al.[33] used combined MPM imaging and biaxial tissue extension to show the in vivo, 3-dimensional architecture of collagen fibre organization in the reticular dermis of men and women, with ages ranging from 36 to 75 years. The tissue was collected during reconstructive surgery. The technique allowed detailed imaging of fibres in situations ranging from tight packing of intertwined fibres to extended or expanded conformation. They showed that in the reticular dermis there are relatively large collagen fibres with a distinctive wavy morphology, densely packed but still distinctly visible as intertwining structures with horizontal laminar organization They further showed that the structure of the dermis varies by depth, e.g., collagen fibres are thicker in the deep reticular dermis and are more densely packed in the middle dermal zone. These insights are advancing our understanding of the fundamental mechanisms underlying the role of collagen in determining the pliability of human skin.
Collagen production through the lifestages
Collagen production first begins in utero at about the 5th week of the first trimester of pregnancy, at which time fine collagen fibrils can be observed in the developing foetus[34]. During subsequent development collagen matrix increases and is associated with larger fibrils being assembled into larger bundles. As early as 15 weeks into gestation distinct regions of papillary and reticular dermis can be distinguished. Type I collagen content is approximately 70%-75%, compared to type III collagen which is approximately 18%-21%, of the total of collagen content at all gestational ages. This level of Type I collagen is lower than measured in adult skin (~ 85%-90%), whereas the Type III is higher than that observed in adult skin (~ 8%-11%). It is believed that this difference reflects the higher requirements of collagen production to support the developing vasculature and innervation of the foetus. The activities of the enzymes required to synthesise collagen fibres have been reported to vary with age, e.g., the enzyme activities of hydroxylases and glucosyltransferase were expressed maximally in foetal skin, retaining a higher level of activity in the skin of young children compared to adults[35].
During childhood, through prepubertal growth stages and pubertal changes caused by the production of sex hormones in adolescents, there is rapid and extensive collagen turnover. The majority of publications on collagen production in prepubertal and pubertal adolescents is concerned with Type I collagen used as the organic support matrix which is mineralized for the development of bone. Collagen is constantly being synthesised, laid down in the ECM, only to be degraded by enzymes, in particular the MMPs, in a balanced cycle which allows growth. This turnover of collagen is rapid during development and then quiescent during adult years but increases again in later life to compensate for cumulative deleterious damage associated with chrono-ageing and photo-ageing[36,37]. Thus, collagen synthesis and degradation are precisely controlled and biochemically complex processes, which is pivotal to tissue development, tissue repair following damage and tissue maintenance in varied anatomical and physiological systems.
Skin ageing results from a series of divergent processes which affect many constituents of the skin and hence its appearance. There are two primary skin ageing mechanisms, referred to as intrinsic and extrinsic. These intrinsic processes are controlled predominantly by genetic and hormonal variations, whereas extrinsic components include smoking, alcohol consumption, chronic sun exposure, stress, and several other factors. Extrinsically aged skin is characterised by several clinical manifestations including the presence of fine lines and increased wrinkle formation, reduced recoil capacity, increased fragility of the skin and altered melanogenesis and skin pigmentation.
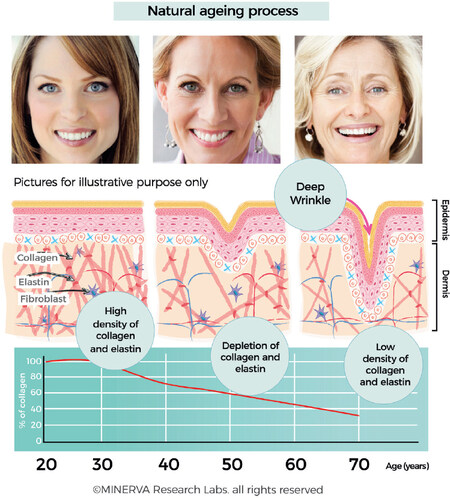
The proportion of the collagen types in skin change with age[38]. Young skin is composed of 80% type I collagen and about 15% collagen type III[39]. With age, the ability to replenish collagen naturally decreases by about 1.0%-1.5% per year. This decrease in collagen is one of the characteristic hallmarks associated with the appearance of fine lines and deeper wrinkles [Figure 5]. Moreover, deep inside in the dermis, fibrillar collagens, elastin fibres and hyaluronic acid, which are the major components of the extracellular matrix, undergo distinct structural and functional changes.
Figure 5. Collagen content of skin is maximal between the 2nd and 3rd decade, after which there is a slow depletion and loss of collagen (and associated ECM components such as elastin and GAGs). The loss of collagen is clearly correlated with changes to appearance attributes which are typically referred to as fines lines and wrinkles. (By permission of MINERVA Research Labs Ltd - London)
Collagen and elastin are stable proteins with a half-life measured in years (t1/2 for skin collagen is approximately 15 years) and hence are predisposed to long term cellular stress[40]. In considering the collagen bundle it is obvious that the bulk of the collagen protein is inaccessible due to the close packing of individual fibrils. Even in the outer sheath the proteins are chemically cross-linked to the fibres inside the bundle and are thus not readily cleaved by proteases. This highlights the importance of MMP enzymes which can cleave the collagen triple helix and make the fibre accessible to degradation enzymes and cellular recycling[41].
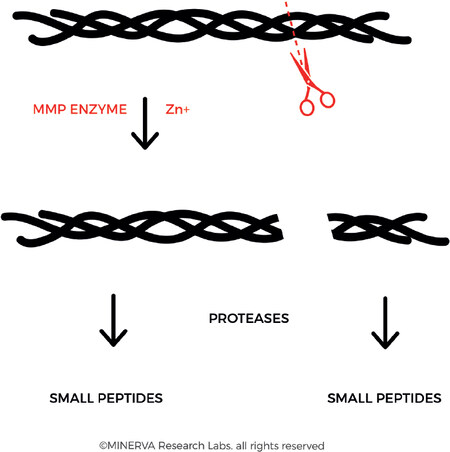
In the family of MMPs, it is the collagenases that are required to carry out the first degradation step, in which the fibres are cleaved into characteristic ¼ and ¾ fragments [Figure 6]. According to the Lauer-Fields model, cleavage occurs at the border of a tight triple helix region (high in imino acid content) and a loose triple helix region (low in imino acid content), where the enzyme can unwind the triple helix strands and initiate hydrolysis of the individual strands[42]. Following this first step, other proteinases continue the degradation of the collagen fibres, including gelatinase (MMP-2), serine proteinases, cysteine proteinases, and aspartic proteinases.
Figure 6. Collagenase (also referred to as Matrix metalloproteinase, MMP) binds and locally unwinds the triple-helical structure allowing subsequent hydrolysis of the exposed peptide bonds. The enzyme preferentially interacts with the α2(I) chain of type I collagen and cleaves the 3 α chains in succession. This results in the derivation of the characteristic 3/4 and 1/4 fragments. (By permission of MINERVA Research Labs Ltd - London)
Collagen fibres accumulate damage over time and this decreases their ability to function correctly. Intrinsically aged skin is generally characterised by dermal atrophy with reduced density of collagen fibres, elastin, and hyaluronic acid[43]. In addition to reduced density, the collagen and elastin fibres can be observed to be disorganized and abnormal in aged skin compared to young and healthy skin[44]. As collagen levels start to decline, the collagen structure becomes more fragile and brittle leading to a weakening of the skin’s structural support. The skin loses volume and firmness and starts to thin and wrinkle. The reduction in collagen production also coincides with a loss of hyaluronic acid further impacting on the hydration and suppleness of the skin.
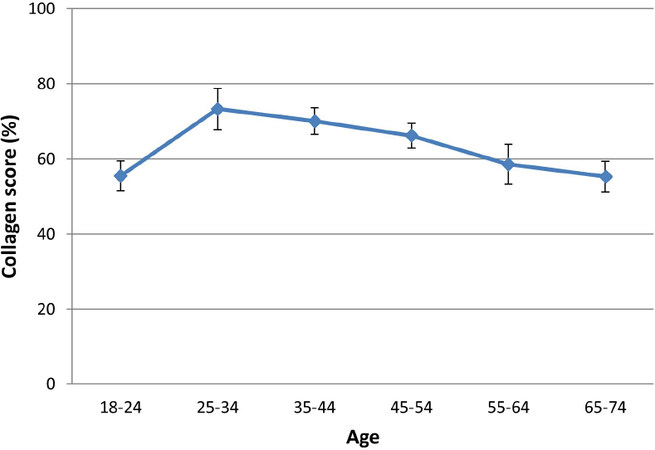
In a study published by Sibilla et al.[45], the researchers reported a peak in collagen content for subjects between 25-34 years old, followed by a gradual decline equating to an approximate 25% decrease over 4 decades (Percent collagen score 73.28 ± 14.3 at age 25-34 vs. 55.3 ± 13.1 at age 65-74, n = 64, Figure 7). This decline in collagen in aged skin has been measured using various methodological approaches, which are generally in good agreement and support the hypothesis of collagen loss being a key determinant of age-related deterioration of skin appearance[46,47].
Figure 7. Collagen content in skin tends to increase until approximately the mid-20s. Thereafter, there is a progressive loss of collagen through the decades. (By permission of MINERVA Research Labs Ltd - London)
The endocrine system and hormonal effects on skin collagen
Changes in hormones levels associated with chrono-ageing affects different parts of the body in various ways. With hormonal changes during teenage years and puberty many adolescents experience acne, caused by an interaction of hormones, sebum-based oils, and resident bacteria and associated with inflammation, redness, and spots. Acne can be severe in clinical presentation and can cause scarring of the skin. Scarring of skin requires tissue remodelling, including remodelling of the collagen-based ECM, to repair the damage associated with long term inflammation and tissue atrophy.
An increase in hormonal levels is accompanied by increased activity of sebaceous glands, with an increase specially in androgens, resulting in an excess of sebum produced in skin. During early adult life, hormone levels start decreasing, thus acne symptoms starts to lessen. However, facial lesions can affect people throughout their entire adulthood[48,49]. Women may repeatedly suffer acne in adulthood as this may occur with their menstrual period, especially for those who suffer from PCOS (Polycystic Ovarian Syndrome). This hormonal disorder affects the menstrual cycle and can increase the severity of acne. Most women suffer from acne disorders until menopause period when levels of oestrogen start decreasing rapidly[50].
Several studies have suggested that following a healthy and balanced diet can help treat acne, especially food rich in vitamin A, vitamin D, vitamin B3 and vitamin B5 which help reduce inflammation, lesions, and scars[51]. Niacinamide, or vitamin B3, is commonly used to reduce swelling and redness due to its anti-inflammatory properties and also helps regulate the amount of oil produced by sebaceous glands in skin. Furthermore, niacinamide regulates skin tone, helps minimise marks on the skin and reduce appearance of hyperpigmentation[52]. Clinical studies using daily oral supplementation containing pantothenic acid in healthy human adults with mild and moderate acne has shown the reduction of total facial acne and blemishes after 8- and 12-weeks respectively versus placebo control[53,54]. A deficiency in Vitamin D (25 hydroxyvitamin D3) was shown to correlate with increased severity of acne lesions, which could be mitigated by supplementation with oral cholecalciferol at 1000 IU/day for 2 months[55].
Changes in collagen synthesis and degradation during pregnancy and postpartum have been instrumental in understanding collagen turnover in ECM remodelling. Collagen and elastin undergo a marked increase in pregnancy followed by a rapid decrease during involution[56]. Pregnant women can experience many integumentary distortions, including skin stretch and hair loss (which can be pre- or post-partum) whereas post-partum skin elasticity needs to be restored by helping to tighten the skin on the abdominal area. As pregnancy progresses, the skin around the stomach area, hips, thighs, and breast expands and many women develop stretch marks. Pregnancy stretch marks (striae gravidarum) are common at later stages affecting up to 90% of women and depend on the viscoelastic tension forces of the skin. During pregnancy, hormones soften collagen fibres by decreasing the bonding between them and increasing the appearance of stretch marks[57]. Loose skin on the stomach area is very common, and skin may never revert back to its original elasticity.
Other forms of stretch marks (striae distensae; striae rubrae) are lines or streaks across the skin, usually quite narrow and can be pink, red, or purple[58]. They usually start off darker and fade over time leaving pale marks and lines in the skin. The most affected areas are the abdomen, breasts, and thighs. Stretch marks are also caused by sudden growth, weight gain (e.g., obesity) or puberty.
Collagen supplementation during and after pregnancy (in particular during breastfeeding) can be a key beneficial support to the immense amount of changes that the body goes through during that period, supporting a hydrated and more elastic skin architecture, making it healthier and stronger, especially post-partum. It has also multiple benefits for joints, ligaments, muscle which will help carrying the baby during the pregnancy period and help alleviate muscle soreness and injuries.
Since the pioneering work of Albright et al.[59] in 1941 the association between atrophied skin, menopausal status of women and prevalence of osteoporosis have been studied extensively. It has been shown that a decrease in skin thickness and collagen content occurs with decreasing oestrogen concentration[60]. Symptoms associated with the menopause include hot flushes, insomnia, decreased skin elasticity, decreased skin hydration, varicose veins, cellulite and impaired cognitive function. These symptoms can lead to frustration and impact negatively on Quality of Life outcomes. Men, on the other hand, have a gradual decline in testosterone levels (which has less impact on collagen content) and therefore experience less symptoms if compared with women of similar characteristics and age. Several studies support the anti-ageing properties of oestrogens in postmenopausal women showing a positive effect increasing skin collagen content, thickness, elasticity, and hydration as well as improving would healing and reducing wound complications[61,62].
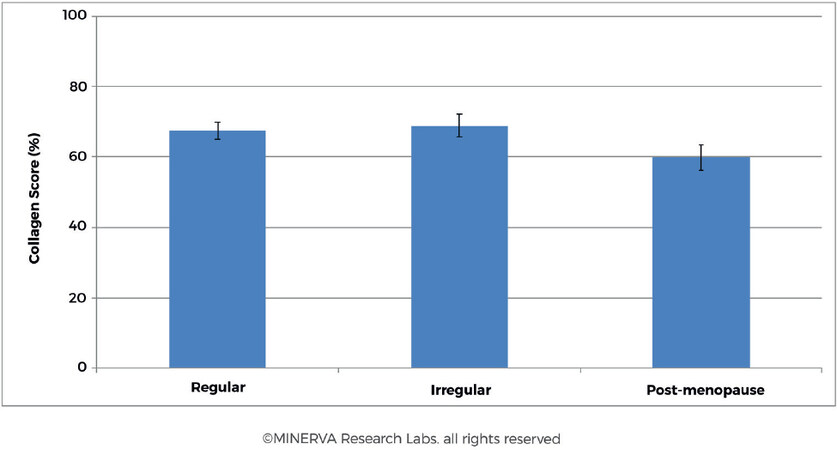
Studying the collagen content of the skin during menopause, an average decline of 2.1% on skin collagen content and 1.13% on skin thickness per each postmenopausal year during the first 15-18 years of post-menopause was observed[63,64]. In a study carried out on 65 women of varying age and menstrual cycle the collagen content was reduced in women of post-menopausal status [Figure 8][45]. Results indicated a decrease in collagen score measured in post-menopausal women (Percent Collagen Score = 59.8 ± 18.1, n = 27) compared to pre-menopausal subjects (Percent Collagen Score = 67.9 ± 12.1, n = 38). Intriguingly there was no direct correlation between women presenting regular or irregular menstrual cycle while carrying out the study (Percent Collagen Score = 67.4 ± 12.5, n = 25 and 68.9 ± 11.7, n = 13, respectively).
Figure 8. For women presenting regular or irregular menstrual cycle there was no discernible difference in collagen content in skin. However, for the post-menopausal cohort the trend shows an approximately 13% decrease in collagen content of the skin. (By permission of MINERVA Research Labs Ltd - London)
Researchers have explored innovative strategies using oestrogen-related treatments to help improve skin conditions[65,66]. Although the effects of oestrogen on the skin are still not fully understood, it is known that, in women declining oestrogen levels are associated with a variety of cutaneous changes, many of which can be reversed or improved by supplementation with estrogenic-like substances. Hormone Replacement Therapy (HRT) is a treatment to relive symptoms of the menopause, usually combining oestrogen and progesterone. It replaces systemic hormones that occur at a lower level as progression of the menopause occurs. The key benefits of HRT are to help restore collagen in skin, relieve hot flushes, reduce night-sweats, control mood swings, decrease vaginal dryness, among others.
HRT oestrogen with or without progesterone has been used to treat menopausal symptoms and to prevent long-term conditions such as osteoporosis and cardiovascular disease. In a randomized placebo-controlled trial were evaluated the effects of genistein on hot flushes in postmenopausal women for 1 year. The flush score decreased by 24% with genistein compared to 54% with synthetic hormone analogues used in HRT[67].
Isoflavones and lignans are the two main groups of phytoestrogens (PE). Isoflavones are polyphenolic compounds that possess both oestrogen-agonist and oestrogen-antagonist properties. Isoflavone compounds, such as genistein and daidzein are mainly found in soybean-based products. Genistein is the most widely studied isoflavone, is an angiogenesis inhibitor and a phytoestrogen with antioxidant properties having beneficial effects on human degenerative diseases. Daidzein, on the other hand, has been shown to increase fibroblast proliferation in fibromuscular coat of the vaginal epithelium and in human skin[68]. In another double-blind placebo-controlled clinical trial, researchers studied the effects of isoflavones on menopausal symptoms, including dry skin, facial hair, libido, and vaginal dryness in postmenopausal women aged 50 to 75 years. Three months of soy supplements containing PE did not provide symptomatic relief compared with placebo[69]. Unfortunately, there are still insufficient data to understand the long-term implications of PE use.
Oxidative damage and repair of collagen
In the ageing process the long-term effects of oxidative damage to cells and tissues is a key mechanism which can be targeted by intervention strategies so that we can attempt to slow the damaging effects of ageing. In this context a disturbance in the balance between the production of reactive oxygen species (ROS) and our cellular protection via antioxidant defences is defined as oxidative stress[11,70]. ROS are a specific subset of free radical species that act by driving several molecular pathways that play important roles in pathologic conditions such as cancer, heart diseases and diabetes. Sun damage (in particular UVA-radiation mechanisms which regulate ROS production) can cause both skin cancer and photo-ageing, affecting the skin through wrinkling, scaling, dryness and mottled hyperpigmentation[71]. The ROS can cause damage to intracellular constituents such as DNA, lipids and proteins. However, the skin possesses defence mechanisms which interact with toxicants and counteract their damaging effect (including both non-enzymatic and enzymatic molecules that function as potent antioxidants). These defences, although highly effective, have limited capacity and can be overwhelmed, especially during ageing, leading to increased ROS levels and to the associated increased risk of dermatological diseases.
Free radical species are defined by the presence of unpaired electrons in the outer shells of the atom, or constituent atoms of molecules[72]. This unstable configuration will seek to find an electron, either to take (in the case of ionic bonds) or to share (in the case of covalent bonds). The high energy free radicals can do a lot of damage to cellular structural components (such as lipid bilayer membranes) or subcellular components (such as proteins, lipids or DNA) that they encounter. These high energy species react rapidly with neighbouring molecular species, and thus have a very short half-life and a low steady state concentration in situ. Free radical Initiation occurs when a high energy event, such as when a UVB or UVA photon strikes a target atom, stripping an electron from an outer shell[73]. Initiation may also occur as a consequence of oxidative metabolism and mitochondrial respiration in the cell. When a free radical reacts with another molecule it in turn generates another free radical, in what is referred to as the Propagation stage. This causes a chain reaction which is inherently dangerous to any biological system. The final stage is referred to as Termination, which ends the chain reaction.
The consequence and impact of ROS depends on the ability of the cell to limit the free radical attack and repair the damage. In the case of DNA, specific enzymes such as NAD-dependent Poly ADP Ribose Polymerase (PARP), can repair the damage to DNA, preventing coding errors or mutations in the genetic code[74]. Lipid turnover is typically high and ensures replacement of lipid peroxides. Protein damage however can be difficult to repair, especially if the turnover rate of the protein is low. However, it is important to note that damage to the collagen protein is likely via an indirect mechanism. The main biological target of free radical damage in the case of proteins is that of sulphydryl-containing species, including the tripeptide glutathione, which has a high sulphydryl content due to the presence of cysteine[75,76]. Glutathione can be recycled using NADPH (the reduced form of nicotinamide adenine dinucleotide phosphate) as a cofactor, thus making it a highly effective free radical scavenger. However, the ensuing oxidative depletion of glutathione and consequent inflammation cascade leads to increased transcription, translation and expression of MMP enzymes which can affect integrity of the ECM[77]. As explained earlier in this review, the matrix metalloproteinase family of enzymes (especially MMP-1 and MMP-3) can degrade collagen fibres leading to a loss of functional ECM.
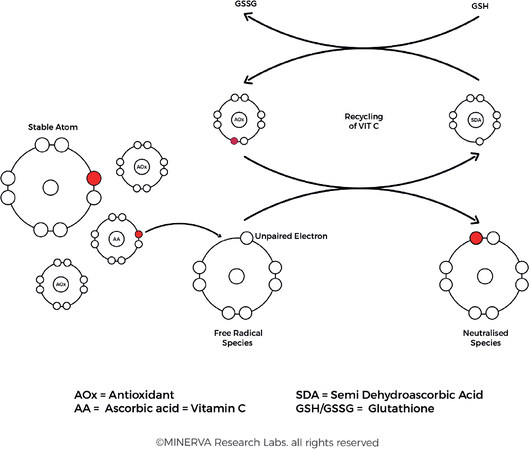
One approach to prevent or treat these ROS-mediated disorders is based on the administration of different antioxidants in an effort to restore homeostasis. Free radical scavengers from dietary and supplemental sources include water soluble ingredients such as Vitamin C (l-ascorbic acid), lipid soluble ingredients such as Vitamin E (d-α-tocopherol), and a vast array of antioxidant species sourced from botanicals, including flavonoids, carotenoids and numerous plant extracts. The antioxidant species protect the cell by neutralizing the free radicals, but in the process themselves become free radical species. However, it is more efficient for the cell to recycle, repair or regenerate small molecular weight species such as ascorbic acid and this effectively keep larger molecular weight structures such as protein, lipids and DNA protected from damage [Figure 9].
Figure 9. Free radical damage occurs when unpaired electrons attack the electrons in the outer shell of a nearby atom. Antioxidants protect the cellular constituents by donating an electron to neutralize the free radical species, after which they can be recycled, repaired, regenerated, or removed. (By permission of MINERVA Research Labs Ltd - London)
Ascorbic acid is capable of interacting with a range of free radical species to facilitate their detoxification. In the process the ascorbic acid itself is converted into a stable ascorbyl free radical, which is a much less reactive species and therefore less likely to cause oxidative damage to cellular components. The ascorbic acid can be recycled via cytosolic glutathione-dependent pathways or membrane-bound NADH-dependent reductase pathways[78]. Vitamin C also is able to preserve the activity of vitamin E by converting the tocopheryl radical back to its native form, restoring the biological activity of the tocopoherol species[79].
It is important to realise that ROS threat to collagen integrity and content in the ECM can be generated through many distinct pathways. In addition to UV-radiation, other mechanisms include generation of Advanced Glycation End products (AGE), Advanced Lipid oxidation End products (ALE), diet and lifestyle, alcohol consumption, smoking or pollution related xenobiotic metabolism which can be associated with production of polycyclic aromatic hydrocarbon species[70].
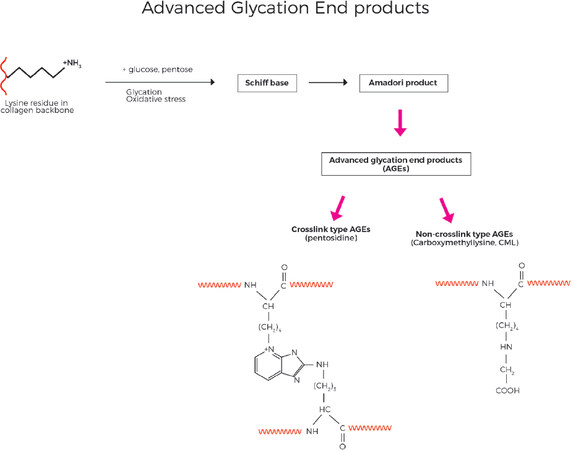
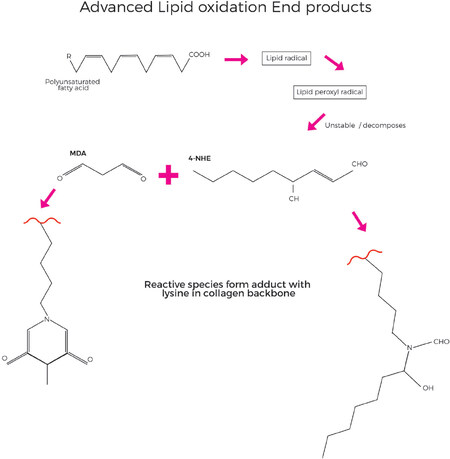
Within the collagen protein backbone, early glycation reactions can occur in which glucose reacts in a non-enzymatic and reversible manner with free amino groups of lysine. Although this reaction is reversible, with cumulative oxidative stress, the combination of glycation and oxidation forms irreversible adducts with the protein which ultimately become AGE, specifically as carboxymethyllysine and pentosidine adducts [Figure 10][40]. In a distinct but related mechanism, ALE involving polyunsaturated fatty acids as a primary target for free radical attack, leads to production of lipid peroxy radicals, lipid hydroperoxides and aldehyde products [Figure 11]. Malondialdehyde (MDA) and 4-hydroxy-2-nonenal (4-HNE) are key lipid oxidation products and can react with free amino groups of the collagen protein, once again predominantly lysine as the other classic amino acids that are susceptible to react with MDA and 4-NHE (histidine and cysteine) are not present in collagen at significant levels[71]. Due to the slow turnover of collagen, the damage can accumulate over years and decades. The cumulative damage of the collagen proteins in the ECM due to ALE and AGE species disrupts their normal structure and metabolism and leads to increased stiffness and rigidity and loss of function.
Figure 10. Advanced Glycation End product (AGE) are formed when sugars such as glucose or pentose react with lysine residues in the collagen backbone, eventually leading to generation of either non-crosslinking type AGE species such as CML, or crosslink type AGE such as pentosidine. (By permission of MINERVA Research Labs Ltd - London)
Figure 11. Advanced Lipid peroxidation End products (ALE) form when polyunsaturated fatty acids are oxidised, forming lipid radical species which can lead to production of reactive substances such as MDA (Malondialdehyde) or 4-HNE (4-hydroxy-2-nonenal), which react with lysine residues in the collagen backbone, leading to cumulative damage over prolonged periods of time. (By permission of MINERVA Research Labs Ltd - London)
Using a well-balanced combination of both water-soluble and lipid-soluble antioxidants in supplements formulated to deliver optimal absorption, vascular distribution and cellular bioavailability, it is possible to delay skin ageing and to improve skin conditions[80].
Anti ageing strategies related to skin collagen
It has been reported that skin health and beauty are principal factors representing overall wellbeing and the associated perception of health in consumers[81]. The distinctions between chrono-aged skin (which tends to be thin, dry and finely wrinkled) and photo-aged skin (which tends to be thickened, hyperpigmented, deeply wrinkled and exhibiting a rough profilometric topography) allows targeted intervention strategies to be devised. The MacArthur Foundation Study of Successful Aging was hailed as the “new gerontology” and advocated the potential for a healthy and engaged old age[82,83]. This is an alternative view to the older “decline and loss” paradigm that views ageing as a series of individual decrements or losses to which both elders and society needed to adapt or adjust. Supporting both of these concepts has been a desire to minimise the visible signs of skin ageing.
Stimulation of collagen production and/or inhibition of collagen degradation can be achieved in several ways, including the use of surgical aesthetic treatments, topical treatments, or use of oral supplements (often referred to as “nutricosmeceuticals”). In a report published by the European Union, the critical importance of nutrition in active and healthy ageing has been clearly described for both macronutrients and micronutrients[84]. The link between nutrition and skin ageing has also been reviewed in detail by Schagen et al.[85]. Of 11 intervention pathways/strategies mentioned in this review, 7 are directly related to collagen content in the skin, underlining the importance of this protein to skin integrity and ageing.
Supplement drinks containing hydrolysed bioactive collagen peptides, in combination with vitamins, minerals and botanical antioxidants are frequently used in nutricosmeceutical products to improve skin elasticity, hydration and visible signs of fine lines and wrinkles[86-89]. Furthermore, studies have reported benefits for nail growth and reduction of the symptoms associated with broken, brittle or split nails[90].
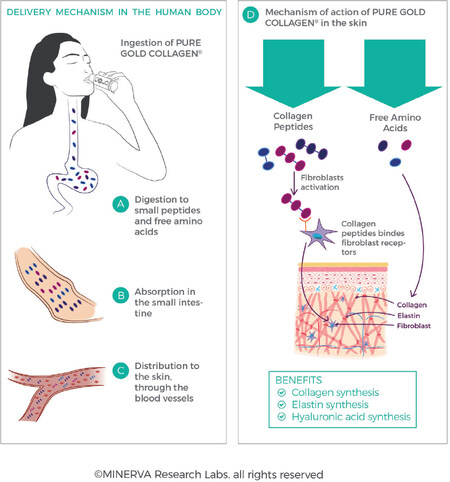
All proteins and peptides need to be hydrolysed in the gut to allow absorption into the bloodstream and transport throughout the body [Figure 12]. Following ingestion, partially hydrolysed collagen peptides in supplements are further digested and hydrolysed in the gut[91]. This is carried out by the action of the acidic environment in the stomach, as well as by the action of specific enzymes in the intestines (trypsin, chymotrypsin, elastase, carboxypeptidase) which break up the collagen peptides into smaller molecular weight fragments. The peptides are progressively broken down as they pass into and through the intestines to yield small peptides (typically di- and tri-peptides) and free amino acids. It has been estimated that 90% of absorbed proteins is represented in the circulatory system as amino acids, where only 10% is present as di- or tri-peptides[92,93]. This enzymatic processing facilitates cellular uptake typically via transporter proteins (such as amino acid cotransporter systems or the low-affinity, high-capacity peptide transporter, PEPT1) to deliver the nutrients from the lumen into the enterocyte cells, and across the basolateral membrane of the enterocyte into the bloodstream.
Figure 12. Collagen protein, whether intact or partially hydrolysed, is enzymatically hydrolysed in the gut to amino acids, dipeptides and tripeptides, which allows for transport across the intestinal wall and into the bloodstream (A,B,C). Perfusion of peptides and amino acids and bioavailability in the ECM allows for stimulation of fibroblasts and collagen synthesis (D). (By permission of MINERVA Research Labs Ltd - London)
From a liquid format, the ingredients are readily absorbed into the bloodstream (typically in about 20 min after ingestion). By comparison, absorption from solid foods can take several hours. Similar to the process for digestion and absorption of proteins, the majority of minerals, vitamins and other nutrients (e.g., sugars, lipids) are absorbed as simple compounds into the bloodstream[94]. From the bloodstream, these ingredients are then distributed throughout the whole body. Perfusion of micronutrients out of capillary loops and into the skin, creates a microenvironment enriched with nutrients which bathes the dermis.
The hydrolysed collagen has 2 distinct, but complimentary, functions. Firstly, the amino acids from hydrolysis of collagen in the GI tract are the building blocks used by the fibroblast cell to make more collagen. As collagen is uniquely rich in glycine, proline (and hydroxyproline, which is derived by post-translational modification during collagen synthesis), this represents an enriched supply of the specific amino acids required to make new collagen fibrils. Secondly, unique oligopeptide sequences, especially dipeptides containing hydroxyproline, are known to stimulate fibroblasts via receptor-mediated activation pathways to induce new collagen fibre synthesis[95]. Although present at lower levels than amino acids, the peptides can stimulate fibroblast receptors and are thus biologically potent even at lower absolute concentrations.
The biological potency and clinical efficacy of hydrolysed collagen can be linked to both its unique amino acid profile and specific oligopeptide sequences, which underlines the key characteristics contributing to the major success of hydrolysed collagen as a supplement for health benefits in the body. Other proteins, for example from casein, peanuts or tofu, have a different amino acid composition and are lower in relative contribution of specific amino acids required for ribosomal synthesis of protein (which uses the enzyme, aminoacyl tRNA synthase, to attach the appropriate amino acid via an ester bond). The appropriate tRNA complex is used to synthesise the protein on the ribosomes of the endoplasmic reticulum of the cell[96,97]. As collagen is the most abundant ECM protein the tRNA species need to be enriched with the appropriate proteinogenic precursors for collagen synthesis. However, this situation is complicated by the fact that amino acids such as glutamine, glutamate and aspartate are highly metabolized in the gut and do not appear in appreciable amounts in the bloodstream. A comprehensive review by Albaugh et al.[98] explores the research whereby supplementation with specific amino acids such as proline was carried out in order to stimulate collagen synthesis, but proof of superior efficacy to hydrolysed collagen requires further studies.
During digestion many di- and tri-peptides are produced in situ. In principle, from 18 proteinogenic amino acids it is possible to derive 324 dipeptides or 5,832 tripeptides. Even if we account for an enrichment of glycine, proline and hydroxyproline species, the number of peptides which can potentially stimulate fibroblasts to synthesis new collagens is too large to test in vivo. Research has shown that significant amounts of the di- and tri-peptide species, Pro-Hyp, Ala-Hyp, Ala-Hyp-Gly, Pro-Hyp-Gly, Leu-Hyp, Ile-Hyp and Phe-Hyp were measurable in human blood following oral ingestion of different collagen hydrolysates[95-100]. Some of these di- or tri-peptides have been shown to stimulate fibroblasts in vitro[101,102]. However, superior efficacy of individual or synthetic peptides over the complex mix of oligopeptides generated by digestion of collagen has not been shown to date. Until such proof is provided, it is a better option to continue to use hydrolysed collagen, as processed through the digestive system, as a source of fibroblast-stimulating peptides. Likewise, topical products using collagen peptides cannot provide this wide spectrum of bioactive peptides, in addition to the problems of transcutaneous absorption of oligopeptides across the stratum corneum being limited.
The expression “Beauty Vitamins” has been applied to several vitamins, but the importance of Vitamin C to skin is unique in that ascorbic acid can act as a co-factor to several enzymes in the production of collagen, in addition to its role as an antioxidant in protecting against free radical damage. The importance of vitamin C in the production of functional collagen fibres has been shown to be dependent on its use as a cofactor in hydroxylation of proline residues in procollagen (which stabilises the triple helix structure) and lysine residues (which are used to cross-link fibres imparting structural rigidity and stability). Hydroxylation is catalysed by Fe(II)-dependent dioxygenases in the case of prolyl and lysyl hydroxylases[103]. Collagen prolyl-4-hydroxylase enzymes (C-P4H) catalyse the formation of 4-hydroxyproline (4-Hyp) on collagens by modifying proline residues in the Y position (of the X-Y-Gly sequence), in a process that requires Fe2+, molecular oxygen and ascorbic acid[104]. This modification takes place in the endoplasmic reticulum before collagen triple helix formation. The content of 4-Hyp is a key determinant of the stability of the collagen triple helix, without which conditions such as scurvy can become manifest.
As can be seen in Figure 3, in the endoplasmic reticulum of the fibroblast cell, specific lysine residues are hydroxylated by the lysyl hydroxylase enzyme to form hydroxylysine. Specific hydroxylysine residues of the procollagen peptide can be subject to O-linked glycosylation to either a galactosylhydroxylysine or glucosylgalactosylhydroxylysine by the action of their respective transferase enzymes[105].
Modification of lysine residues are critical to the final step of covalent intramolecular and intermolecular cross-linking, which imparts strength, rigidity and longevity to the collagen fibre [Figure 3][106]. Type I collagen has only 4 locations at which this process occurs, i.e., 2 telopeptide sites at either end of the peptide and 2 triple helix sites along the peptide backbone. There are 2 pathways used for cross-linking of collagen, one based on formation of a lysine-derived aldehyde and the other based on formation of a hydroxylysine-derived aldehyde[25]. The former pathways is key to generation of collagen-based skin ECM.
In the extracellular space, lysine residues of the N- and C- telopeptides can be oxidatively deaminated to produce reactive aldehydes via the activity of lysyl oxidase[105]. Lysyl oxidase is a copper-metalloenzyme requiring vitamin B6 (pyridoxal phosphate) as a cofactor which can convert the amine side chain of lysine (and/or hydroxylysine) into the corresponding aldehyde. These reactive species can then undergo a series of non-enzymatic condensation reactions with hydroxylysine resides along the peptide backbone to form covalent intramolecular and intermolecular cross-links [Figure 3]. Although this sequence of enzymatic modifications at first seems complicated, it is an elegant system that allows the final stages of cross-linking of the large collagen fibers to occur outside the cell and in the ECM, allowing for formation of the large structural protein scaffold and elastic matrix that supports skin.
Skin appearance attributes
As the saying goes “beauty is in the eye of the beholder”, suggesting that it is a subjective, perceptual cognitive process, which is difficult to measure and quantify. However, beauty is a construct of visible and measurable physical features which determine appearance. The taxonomy of appearance traits has been reviewed in detail by Igarashi et al.[107] and can be categorized based on skin components measured across 3 distinct scales, the micro, meso and the macro scale, in order of increasing size. Each scale can be characterised in greater details by using high resolution detection methods which can be further correlated to physiology and anatomy. Thus, skin appearance attributes can be viewed at several distinct levels, each related to beauty depending on the outcomes applied.
The micro scale is determined by various cellular elements and skin layers, in which the sizes of these subcellular organelles are typically very small and thus barely visible to the naked eye of the observer. This includes cells and fibres and their optical interactions with incident light are dependent on optical phenomena such as scattering and absorption. A key measurable parameter is the refractive indices of the elements, e.g., collagen fibres in the ECM interact with an incident beam of light to cause a strong scattering of the photons. The cellular level elements include the epidermis, dermis and subcutis.
Skin and skin features constitute the meso scale. At this scale, the components become visible to the naked eye. The visual properties of these components are mainly determined by the optical phenomena that are induced by finer scale components. Skin is composed of outer corneum layers, skin surface lipid, protruding hair follicles, fine lines and deeper wrinkles, the characteristics of which can be further defined by pigments. Other skin features such as hyperpigmented spots (e.g., solar lentigines) and pores (e.g., eccrine glands, sebaceous ducts) also contribute to the overall appearance and perception of the evenness of complexion.
Body regions and body parts are classified as macro scale. The appearance of skin varies across different regions of the body. This is because the physio-anatomical characteristics of the lower-level components can differ significantly from one region of the body to another. The effects of underlying musculoskeletal features are more noticeable, e.g., the lack of features across the torso compared to the varied anatomical structures seen across the face and neck.
In an open-label study on 217 female volunteers, a nutritional supplement (Pure Gold Collagen®) was tested for its ability to reduce the visible signs of ageing and it was compared to the effects of an aesthetic surgical intervention, such as Botox, laser treatment or the use of dermal fillers[108]. The study reported on facial improvements in the nasolabial folds which extend from the side of the nose to the corners of the mouth. These folds typically deepen with age, and as they are more prominent than other facial lines, their depth is a useful parameter for measuring the effect of anti-ageing products. A 24% reduction in the average score from baseline, as determined by an expert visual assessor was reported. In 37% of subjects, a significant improvement in nasolabial fold depth was observed, with a reduction of 44% in average score. Interestingly, a comparable significant decrease in nasolabial fold depth was reported regardless of whether subjects underwent surgical treatment for nasolabial fold area or not. After 60 days, a decrease in nasolabial fold depth was observed in subjects who underwent other cosmetic treat-ments, with Dermatologists reporting a decrease of 15% for laser treatments, 50% for Botox, 28% for fillers, 41% for treatments in the nasolabial fold area, and an 18% and 10% decrease for mesotherapy and dermabrasion, respectively. In the group of subjects who had dermabrasion, facial laser treatment, or Botox in the upper area of their face for glabellar lines or crow’s feet, there was a respective 4%, 12%, and 18% reduction in the visibility of nasola-bial folds. Not surprisingly, the largest reduction in the number of class 2 wrinkles was observed in subjects who had fillers in their nasolabial folds (29%). There was also a reduction in class 2 wrinkles among those who had mesotherapy or platelet-rich plasma therapy (25%). In the group of subjects who had cheek or lip augmentation by fillers, there was a 25% decrease in class 3 wrinkles.
An independent double-blind, randomised, placebo-controlled clinical trial was performed to investigate the effects of a collagen-based supplement (Gold Collagen® Forte) on skin elasticity in subjects who underwent a cosmetic treatment (fillers and Botox in facial areas) and subjects who did not while using this nutraceutical supplement over a period of 90 days[11,109]. The study showed a statistically significant increase in skin elasticity after 90 days of treatment and an increase in skin elasticity was observed singularly both in subjects who underwent a cosmetic treatment and subjects who did not. Moreover, no significant change in skin elasticity was observed in the placebo group, both in subjects who underwent or did not undergo a cosmetic treatment. The increase in skin elasticity suggests that this functional food supplement, containing collagen peptides among other active ingredients, has an effect in restoring the correct levels of extracellular matrix proteins such as collagen and elastin. Also, the results revealed a reduction in solar elastosis and in hyperkeratosis in the dermis.
Future perspectives and opportunities
Future possibilities for improving collagen synthesis, fibril formation, ECM integrity and skin ageing depend on the on the activity of the fibroblast cell. However, with time, wear and tear, oxidative damage and other cellular influences, the fibroblast becomes senescent, i.e., loses the ability to replicate (replicative senescence). As the fibroblast ages, there is a decrease in telomeres (telomeres are DNA tandem repeats found at the end of chromosomes and known to shorten with each progressive cycle of cell division). Although they remain metabolically active, the senescent fibroblasts experience a decline in their normal cellular functions. Inflammation and cumulative damage are associated with increased telomere shortening, which can be used as a biomarker of cellular senescence, genomic instability and cell ageing in skin of older individuals[110]. This leads to a situation where the skin is not capable of efficiently repairing damaged collagen, with a consequent loss of a functional support matrix related to the visible signs of ageing, such as fines lines, wrinkles and sagging.
Both telomere length and telomerase activity can be measured using models of oxidative stress and measuring the protective effects of antioxidants. Telomere length in human dermal fibroblasts was shortened by a single high dosage of UVA radiation in vitro[111]. It is possible that acute photodamage might contribute to early photo-aging in human skin via this mechanism involving telomere shortening. However, it remains to be seen if such mechanisms also are relevant to the in vivo situation.
Finding new mechanisms to deal with fibroblast senescence and new bioactives to impede telomere loss or repair the DNA damage is an exciting new area of research that may well offer new treatments in the fight against skin ageing.
Conclusion
Collagens are a diverse family of ubiquitous proteins with a wide range of cellular and extracellular functions, supporting cell signalling, proliferation, differentiation, and structural integrity of connective tissues. As the main protein found in the extracellular matrix of skin and bone, Type I collagen represents the most abundant collagen found in the body. Collagen fibres can persist in skin for years but are subject to cumulative damage over a lifetime. The loss of function seen with both chrono-ageing and photo-ageing has led to a multitude of strategies to repair and replace collagen, prevent damage to collagen, provide vitamins and minerals to support biochemical and physiological manipulation of collagen turnover, and optimise interactions with other essential components of the ECM, such as elastin and GAGs. Cosmetic surgery and topical interventions are important strategies in the fight against the visible signs of ageing, especially in cases where visible results are required in a short period of time. In the long term, anti-ageing benefits can be enhanced by the addition of expertly crafted nutricosmeceutical supplements, with the overall aim to rejuvenate ageing or damaged skin, improve skin integrity, appearance, beauty, and support personal wellbeing and vitality.
Declarations
AcknowledgementsFor support in artworks, graphics and design the authors thank Santiago Cornejo, Patricia Delgado and Anita Hoxha. Thanks to Sara Sibilla for help in preparation of the manuscript.
Authors’ ContributionsSections including abstract, introduction, oxidative damage, anti-ageing strategies and future perspectives: Reilly DM
Sections including lifestages, endocrine, skin appearance and conclusion: Lozano J
Availability of data and materialsNot applicable.
Financial support and sponsorshipNot applicable.
Conflicts of interestBoth authors work for Minerva Research labs, which produces collagen-based supplements for skin care and health care.
Ethical approval and consent to participateNot applicable.
Consent for publicationNot applicable.
Copyright© The Author(s) 2021.
REFERENCES
1. Fleischmajer R, Perlish JS, Timpl R. Collagen fibrillogenesis in human skin. Ann N Y Acad Sci 1985;460:246-57.
2. Sibilla S, Godfrey M, Brewer S, Budh-Raja A, Licia G. An overview of the beneficial effects of hydrolysed collagen as a nutraceutical on skin properties: scientific background and clinical studies. Open Nutraceuticals J 2015;8:29-42.
3. Nolte SV, Xu W, Rennekampff HO, Rodemann HP. Diversity of fibroblasts-a review on implications for skin tissue engineering. Cells Tissues Organs 2008;187:165-76.
5. Weihermann AC, Lorencini M, Brohem CA, de Carvalho CM. Elastin structure and its involvement in skin photoageing. Int J Cosmet Sci 2017;39:241-7.
6. Waller JM, Maibach HI. Age and skin structure and function, a quantitative approach (II): protein, glycosaminoglycan, water, and lipid content and structure. Skin Res Technol 2006;12:145-54.
7. Bell E, Ivarsson B, Merrill C. Production of a tissue-like structure by contraction of collagen lattices by human fibroblasts of different proliferative potential in vitro. Proc Natl Acad Sci U S A 1979;76:1274-8.
8. Ogawa R, Hsu CK. Mechanobiological dysregulation of the epidermis and dermis in skin disorders and in degeneration. J Cell Mol Med 2013;17:817-22.
9. Tracy LE, Minasian RA, Caterson EJ. Extracellular matrix and dermal fibroblast function in the healing wound. Adv Wound Care (New Rochelle) 2016;5:119-36.
10. Song H, Li B. Beneficial effects of collagen hydrolysate: a review on recent developments. Biomed J Sci & Tech Res 2017;1:1-4.
11. Genovese L, Sibilla S. Innovative nutraceutical approaches to counteract the signs of aging. In: Farage MA, Miller K, Maibach H, editors. Textbook of Aging Skin. Berlin Heidelberg: Springer-Verlag; 2015. pp. 1-25.
12. Sikora E, Cieslik E, Topolska K. The sources of natural antioxidants. Acta Sci Pol Technol Aliment 2008;7:5-17.
14. Oikarinen A. The aging of skin: chronoaging versus photoaging. Photodermatol Photoimmunol Photomed 1990;7:3-4.
15. Knuutinen A, Kokkonen N, Risteli J, et al. Smoking affects collagen synthesis and extracellular matrix turnover in human skin. Br J Dermatol 2002;146:588-94.
16. Edgar S, Hopley B, Genovese L, Sibilla S, Laight D, Shute J. Effects of collagen-derived bioactive peptides and natural antioxidant compounds on proliferation and matrix protein synthesis by cultured normal human dermal fibroblasts. Sci Rep 2018;8:10474.
17. Fligiel SE, Varani J, Datta SC, Kang S, Fisher GJ, Voorhees JJ. Collagen degradation in aged/photodamaged skin in vivo and after exposure to matrix metalloproteinase-1 in vitro. J Invest Dermatol 2003;120:842-8.
20. Kadler KE, Baldock C, Bella J, Boot-Handford RP. Collagens at a glance. J Cell Sci 2007;120:1955-8.
22. Persikov AV, Ramshaw JA, Brodsky B. Prediction of collagen stability from amino acid sequence. J Biol Chem 2005;280:19343-9.
23. Engel J, Prockop DJ. The zipper-like folding of collagen triple helices and the effects of mutations that disrupt the zipper. Annu Rev Biophys Biophys Chem 1991;20:137-52.
24. Bolboaca SD, Jantschi L. Amino acids sequence analysis on collagen. Bulletin Usa Samv-cn 2007;63-4:311-6.
25. Eyre DR, Paz MA, Gallop PM. Cross-linking in collagen and elastin. Annu Rev Biochem 1984;53:717-48.
26. Buehler MJ. Nature designs tough collagen: explaining the nanostructure of collagen fibrils. Proc Natl Acad Sci U S A 2006;103:12285-90.
27. Kadler KE, Holmes DF, Trotter JA, Chapman JA. Collagen fibril formation. Biochem J 1996;316:1-11.
28. Gelse K, Pöschl E, Aigner T. Collagens-structure, function, and biosynthesis. Adv Drug Deliv Rev 2003;55:1531-46.
29. Munakata H, Takagaki K, Majima M, Endo M. Interaction between collagens and glycosaminoglycans investigated using a surface plasmon resonance biosensor. Glycobiology 1999;9:1023-7.
30. Shin JE, Oh JH, Kim YK, Jung JY, Chung JH. Transcriptional regulation of proteoglycans and glycosaminoglycan chain-synthesizing glycosyltransferases by UV irradiation in cultured human dermal fibroblasts. J Korean Med Sci 2011;26:417-24.
31. Longo C, Casari A, Beretti F, Cesinaro AM, Pellacani G. Skin aging: in vivo microscopic assessment of epidermal and dermal changes by means of confocal microscopy. J Am Acad Dermatol 2013;68:e73-82.
32. Wurm EM, Longo C, Curchin C, Soyer HP, Prow TW, Pellacani G. In vivo assessment of chronological ageing and photoageing in forearm skin using reflectance confocal microscopy. Br J Dermatol 2012;167:270-9.
33. Ueda M, Saito S, Murata T, et al. Combined multiphoton imaging and biaxial tissue extension for quantitative analysis of geometric fiber organization in human reticular dermis. Sci Rep 2019;9:10644.
34. Smith LT, Holbrook KA, Madri JA. Collagen types I, III, and V in human embryonic and fetal skin. Am J Anat 1986;175:507-21.
35. Anttinen H, Oikarinen A, Kivirikko KI. Age-related changes in human skin collagen galactosyltransferase and collagen glucosyltransferase activities. Clin Chim Acta 1977;76:95-101.
36. Ryazanov AG, Nefsky BS. Protein turnover plays a key role in aging. Mech Ageing Dev 2002;123:207-13.
37. Fisher GJ, Quan T, Purohit T, et al. Collagen fragmentation promotes oxidative stress and elevates matrix metalloproteinase-1 in fibroblasts in aged human skin. Am J Pathol 2009;174:101-14.
38. Lovell CR, Smolenski KA, Duance VC, Light ND, Young S, Dyson M. Type I and III collagen content and fibre distribution in normal human skin during ageing. Br J Dermatol 1987;117:419-28.
39. Fleischmajer R, MacDonald ED, Perlish JS, Burgeson RE, Fisher LW. Dermal collagen fibrils are hybrids of type I and type III collagen molecules. J Struct Biol 1990;105:162-9.
40. Verzijl N, DeGroot J, Thorpe SR, et al. Effect of collagen turnover on the accumulation of advanced glycation end products. J Biol Chem 2000;275:39027-31.
41. Pittayapruek P, Meephansan J, Prapapan O, Komine M, Ohtsuki M. Role of matrix metalloproteinases in photoaging and photocarcinogenesis. Int J Mol Sci 2016;17:868.
42. Lauer-Fields JL, Juska D, Fields GB. Matrix metalloproteinases and collagen catabolism. Biopolymers 2002;66:19-32.
43. El-Domyati M, Attia S, Saleh F, et al. Intrinsic aging vs. photoaging: a comparative histopathological, immunohistochemical, and ultrastructural study of skin. Exp Dermatol 2002;11:398-405.
44. Fisher GJ, Wang ZQ, Datta SC, Varani J, Kang S, Voorhees JJ. Pathophysiology of premature skin aging induced by ultraviolet light. N Engl J Med 1997;337:1419-28.
45. Sibilla S, Borumand M. Current understanding of the effects of diet on skin health. In: Godfrey M, editor. Losing weight simply and safely. Florence: Officina Editorale Oltrarno; 2014. pp. 241-68.
46. Varani J, Dame MK, Rittie L, et al. Decreased collagen production in chronologically aged skin: roles of age-dependent alteration in fibroblast function and defective mechanical stimulation. Am J Pathol 2006;168:1861-8.
47. Chung JH, Seo JY, Choi HR, et al. Modulation of skin collagen metabolism in aged and photoaged human skin in vivo. J Invest Dermatol 2001;117:1218-24.
48. Knutsen-Larson S, Dawson AL, Dunnick CA, Dellavalle RP. Acne vulgaris: pathogenesis, treatment, and needs assessment. Dermatol Clin 2012;30:99-106. viii-ix
49. Barnes LE, Levender MM, Fleischer AB, Feldman SR. Quality of life measures for acne patients. Dermatol Clin 2012;30:293-300. ix
50. Hall G, Phillips TJ. Estrogen and skin: the effects of estrogen, menopause, and hormone replacement therapy on the skin. J Am Acad Dermatol 2005;53:555-68. quiz 569-72
52. Jerajani HR, Mizoguchi H, Li J, Whittenbarger DJ, Marmor MJ. The effects of a daily facial lotion containing vitamins B3 and E and provitamin B5 on the facial skin of Indian women: a randomized, double-blind trial. Indian J Dermatol Venereol Leprol 2010;76:20-6.
53. Capodice JL. Feasibility, tolerability, safety and efficacy of a pantothenic acid based dietary supplement in subjects with mild to moderate facial acne blemishes. J Cosmet, Dermatol Sci Appl 2012;2:132-5.
54. Yang M, Moclair B, Hatcher V, et al. A randomized, double-blind, placebo-controlled study of a novel pantothenic Acid-based dietary supplement in subjects with mild to moderate facial acne. Dermatol Ther (Heidelb) 2014;4:93-101.
55. Lim SK, Ha JM, Lee YH, et al. Comparison of vitamin D levels in patients with and without acne: a case-control study combined with a randomized controlled trial. PLoS One 2016;11:e0161162.
56. Read CP, Word RA, Ruscheinsky MA, Timmons BC, Mahendroo MS. Cervical remodeling during pregnancy and parturition: molecular characterization of the softening phase in mice. Reproduction 2007;134:327-40.
57. Torres J, Faris I, Callejas A. Histobiomechanical remodeling of the cervix during pregnancy: proposed framework. Math Probl Eng 2019;2019:1-11.
59. Albright F, Smith PH, Richardson AM. Postmenopausal osteoporosis: its clinical features. J Am Med Assoc 1941;116:2465-74.
60. Stevenson S, Thornton J. Effect of estrogens on skin aging and the potential role of SERMs. Clin Interv Aging 2007;2:283-97.
62. Verdier-Sévrain S, Bonté F, Gilchrest B. Biology of estrogens in skin: implications for skin aging. Exp Dermatol 2006;15:83-94.
63. Brincat M, Kabalan S, Studd JW, Moniz CF, de Trafford J, Montgomery J. A study of the decrease of skin collagen content, skin thickness, and bone mass in the postmenopausal woman. Obstet Gynecol 1987;70:840-5.
65. Castelo-Branco C, Duran M, González-Merlo J. Skin collagen changes related to age and hormone replacement therapy. Maturitas 1992;15:113-9.
66. Brincat M, Versi E, Moniz CF, Magos A, de Trafford J, Studd JW. Skin collagen changes in postmenopausal women receiving different regimens of estrogen therapy. Obstet Gynecol 1987;70:123-7.
67. Crisafulli A, Marini H, Bitto A, et al. Effects of genistein on hot flushes in early postmenopausal women: a randomized, double-blind EPT- and placebo-controlled study. Menopause 2004;11:400-4.
68. Fritsch H, Hoermann R, Bitsche M, Pechriggl E, Reich O. Development of epithelial and mesenchymal regionalization of the human fetal utero-vaginal anlagen. J Anat 2013;222:462-72.
69. Kotsopoulos D, Dalais FS, Liang YL, McGrath BP, Teede HJ. The effects of soy protein containing phytoestrogens on menopausal symptoms in postmenopausal women. Climacteric 2000;3:161-7.
70. Kandola K, Bowman A, Birch-Machin MA. Oxidative stress-a key emerging impact factor in health, ageing, lifestyle and aesthetics. Int J Cosmet Sci 2015;37 Suppl 2:1-8.
71. Lee J, Koo N, Min DB. Reactive oxygen species, aging, and antioxidative nutraceuticals. Compr Rev Food Sci Food Saf 2004;3:21-33.
72. Wu D, Cederbaum AI. Alcohol, oxidative stress, and free radical damage. Alcohol Res Health 2003;27:277-84.
73. Tyrrell RM. Ultraviolet radiation and free radical damage to skin. Biochem Soc Symp 1995;61:47-53.
74. Javle M, Curtin NJ. The role of PARP in DNA repair and its therapeutic exploitation. Br J Cancer 2011;105:1114-22.
75. Webster NR, Nunn JF. Molecular structure of free radicals and their importance in biological reactions. Br J Anaesth 1988;60:98-108.
76. Jocelyn PC. Biochemistry of the SH group: the occurrence, chemical properties, metabolism and biological function of thiols and disulphides. In Oxidation of Thiols. London: Academic Press; 1972. pp. 94-115.
78. Warren JJ, Mayer JM. Tuning of the thermochemical and kinetic properties of ascorbate by its local environment: solution chemistry and biochemical implications. J Am Chem Soc 2010;132:7784-93.
79. Traber MG, Stevens JF. Vitamins C and E: beneficial effects from a mechanistic perspective. Free Radic Biol Med 2011;51:1000-13.
80. Carlsen MH, Halvorsen BL, Holte K, et al. The total antioxidant content of more than 3100 foods, beverages, spices, herbs and supplements used worldwide. Nutr J 2010;9:3.
81. Ganceviciene R, Liakou AI, Theodoridis A, Makrantonaki E, Zouboulis CC. Skin anti-aging strategies. Dermatoendocrinol 2012;4:308-19.
84. Mak TN, Caldeira S. The role of nutrition in active and healthy ageing: for prevention and treatment of age-related diseases: evidence so far. Available from: https://publications.jrc.ec.europa.eu/repository/handle/JRC90454. [Last accessed on 26 Jul 2019].
85. Schagen SK, Zampeli VA, Makrantonaki E, Zouboulis CC. Discovering the link between nutrition and skin aging. Dermatoendocrinol 2012;4:298-307.
86. Asserin J, Lati E, Shioya T, Prawitt J. The effect of oral collagen peptide supplementation on skin moisture and the dermal collagen network: evidence from an ex vivo model and randomized, placebo-controlled clinical trials. J Cosmet Dermatol 2015;14:291-301.
87. Béguin A. A novel micronutrient supplement in skin aging: a randomized placebo-controlled double-blind study. J Cosmet Dermatol 2005;4:277-84.
88. Borumand M, Sibilla S. A study to assess the effect on wrinkles of a nutritional supplement high dosage of hydrolysed collagen. Cosmeceuticals 2014;3:93-6.
89. Borumand M, Sibilla S. Effects of a nutritional supplement containing collagen peptides on skin elasticity, hydration and wrinkles. J Med Nutr Nutraceut 2015;4:47-53.
90. Hexsel D, Zague V, Schunck M, Siega C, Camozzato FO, Oesser S. Oral supplementation with specific bioactive collagen peptides improves nail growth and reduces symptoms of brittle nails. J Cosmet Dermatol 2017;16:520-6.
91. Kiela PR, Ghishan FK. Physiology of intestinal absorption and secretion. Best Pract Res Clin Gastroenterol 2016;30:145-59.
92. Daniel H. Molecular and integrative physiology of intestinal peptide transport. Annu Rev Physiol 2004;66:361-84.
93. Miner-Williams WM, Stevens BR, Moughan PJ. Are intact peptides absorbed from the healthy gut in the adult human? Nutr Res Rev 2014;27:308-29.
94. Goodman BE. Insights into digestion and absorption of major nutrients in humans. Adv Physiol Educ 2010;34:44-53.
95. Kleinnijenhuis AJ, van Holthoon FL, Maathuis AJH, et al. Non-targeted and targeted analysis of collagen hydrolysates during the course of digestion and absorption. Anal Bioanal Chem 2020;412:973-82.
98. Albaugh VL, Mukherjee K, Barbul A. proline precursors and collagen synthesis: biochemical challenges of nutrient supplementation and wound healing. J Nutr 2017;147:2011-7.
99. Iwai K, Hasegawa T, Taguchi Y, et al. Identification of food-derived collagen peptides in human blood after oral ingestion of gelatin hydrolysates. J Agric Food Chem 2005;53:6531-6.
100. Ohara H, Matsumoto H, Ito K, Iwai K, Sato K. Comparison of quantity and structures of hydroxyproline-containing peptides in human blood after oral ingestion of gelatin hydrolysates from different sources. J Agric Food Chem 2007;55:1532-5.
101. Postlethwaite AE, Seyer JM, Kang AH. Chemotactic attraction of human fibroblasts to type I, II, and III collagens and collagen-derived peptides. Proc Natl Acad Sci U S A 1978;75:871-5.
102. Ohara H, Ichikawa S, Matsumoto H, et al. Collagen-derived dipeptide, proline-hydroxyproline, stimulates cell proliferation and hyaluronic acid synthesis in cultured human dermal fibroblasts. J Dermatol 2010;37:330-8.
103. Myllyharju J, Kivirikko KI. Collagens, modifying enzymes and their mutations in humans, flies and worms. Trends Genet 2004;20:33-43.
104. Rappu P, Salo AM, Myllyharju J, Heino J. Role of prolyl hydroxylation in the molecular interactions of collagens. Essays Biochem 2019;63:325-35.
105. Yamauchi M, Sricholpech M. Lysine post-translational modifications of collagen. Essays Biochem 2012;52:113-33.
106. Miller EJ. Collagen Chemistry. In: Piez K, Reddi AH, editors. Extracellular matrix biochemistry. Elsevier; 1984. pp. 41-78.
107. Igarashi T, Nishino K, Nayar S. The appearance of human skin. Foundations and Trends in Computer Graphics and Vision 2007;3:1-95.
108. Borumand M, Sibilla S. Daily consumption of the collagen supplement Pure Gold Collagen® reduces visible signs of aging. Clin Interv Aging 2014;9:1747-58.
109. Genovese L, Corbo A, Sibilla S. An insight into the changes in skin texture and properties following dietary intervention with a nutricosmeceutical containing a blend of collagen bioactive peptides and antioxidants. Skin Pharmacol Physiol 2017;30:146-58.
110. Kosmadaki MG, Gilchrest BA. The role of telomeres in skin aging/photoaging. Micron 2004;35:155-9.
Cite This Article
Export citation file: BibTeX | RIS
OAE Style
Reilly DM, Lozano J. Skin collagen through the lifestages: importance for skin health and beauty. Plast Aesthet Res 2021;8:2. http://dx.doi.org/10.20517/2347-9264.2020.153
AMA Style
Reilly DM, Lozano J. Skin collagen through the lifestages: importance for skin health and beauty. Plastic and Aesthetic Research. 2021; 8: 2. http://dx.doi.org/10.20517/2347-9264.2020.153
Chicago/Turabian Style
Reilly, David M., Jennifer Lozano. 2021. "Skin collagen through the lifestages: importance for skin health and beauty" Plastic and Aesthetic Research. 8: 2. http://dx.doi.org/10.20517/2347-9264.2020.153
ACS Style
Reilly, DM.; Lozano J. Skin collagen through the lifestages: importance for skin health and beauty. Plast. Aesthet. Res. 2021, 8, 2. http://dx.doi.org/10.20517/2347-9264.2020.153
About This Article
Special Issue
Copyright
Data & Comments
Data
 Cite This Article 1263 clicks
Cite This Article 1263 clicks









Comments
Comments must be written in English. Spam, offensive content, impersonation, and private information will not be permitted. If any comment is reported and identified as inappropriate content by OAE staff, the comment will be removed without notice. If you have any queries or need any help, please contact us at support@oaepublish.com.