A comparative study to evaluate the effect of limited access dressing on diabetic ulcers
Abstract
Aim: Emerging evidence favors the important role of antioxidants, matrix metalloproteinases (MMPs) and nitric oxide (NO) in the healing of diabetic wounds. There is a lack of substantial evidence regarding the effects of negative pressure on antioxidants, MMPs and NO in chronic wounds associated with diabetes.
Methods: A total of 55 type 2 diabetic patients with leg ulcers were divided into 2 groups: a limited access dressing (LAD) group (n = 27) and a conventional dressing group (n = 28). Levels of hydroxyproline, total protein, MMP-2 and MMP-9, NO and antioxidants including reduced glutathione (GSH), and the oxidative biomarker malondialdhyde (MDA) were measured in the granulation tissue at days 0 and 10. Changes in levels between the LAD and conventional groups were determined by the student's t-test.
Results: After 10 days of treatment, the LAD vs. conventional dressing group showed increases in the levels of hydroxyproline (mean ± SD = 55.2 ± 25.1 vs. 29.2 ± 1; P < 0.05), total protein (12.8 ± 6.5 vs. 8.34 ± 3.2; P < 0.05), NO (1.13 ± 0.52 vs. 0.66 ± 0.43; P < 0.05), GSH (7.0 ± 2.4 vs. 6.6 ± 2.2; P < 0.05) and decreases in MMP-2 (0.47 ± 0.33 vs. 0.62 ± 0.30; P < 0.05), MMP-9 (0.32 ± 0.20 vs.0.53 ± 0.39; P < 0.05) and MDA (6.8 ± 2.3 vs. 10.4 ± 3.4; P < 0.05).
Conclusion: When compared to conventional dressings, LAD induces biochemical changes by significantly increasing the levels of hydroxyproline, total protein, NO and antioxidants levels, and significantly reducing MMPs (MMP-2 and MMP-9) and an oxidative biomarker in diabetic wounds. These biochemical changes are thought to favor diabetic wound healing.
Keywords
Introduction
Wound healing is a self-regulated physiological process of cell regeneration which occurs without any external stimuli. This is accomplished by the stages of fibroplasia, angiogenesis and migration of fibroblasts, endothelial and epithelial cells, finally leading to the wound contraction.[1] Inflammation is a vital and protective response instigated by injured cells at the wound site which begins the process of tissue repair.[2] In response to inflammation, reactive oxygen species (ROS), such as free radicals (superoxide anion radical: O2-) and nonradical hydrogen peroxide (H2O2), are generated continuously until inflammation subsidies.[3] Free radicals and their scavenging systems play an important role in normal and delayed wound healing.[4,5]
Delayed healing of diabetic wounds is characterized by an increase in matrix metalloproteinases (MMPs), a decrease in the tissue inhibitors of metalloproteinases (TIMPs),[6] and an altered magnitude of free radical generation and disposal.[7] An imbalance between oxidant and antioxidant defense mechanisms leads to oxidative stress resulting in lipid peroxidation, DNA damage and inactivation of free radical scavenger enzymes.[8] This leads to tissue damage and impairs the healing process in diabetic wounds with reduced angiogenesis, altered proliferation of fibroblasts, reduced fibroblast migration, inadequate collagen deposition, advanced glycation, and abnormal mitochondrial function.[9-11]
Nitric oxide (NO) is a mediator which that plays an important role in wound healing and has been implicated in diabetic wounds. Reduced levels can cause alterations in vascular permeability and a reduction of capillary flow, causing oxidative stress.[12] Antioxidants such as reduced glutathione (GSH), glutathione peroxidase (GPx), catalase (CAT), and thiol (-SH) prevent the generation and action of ROS. Hence, antioxidants that provide potential mechanisms for wound healing and can ameliorate diabetic complications by both significantly preventing tissue damage and stimulating the wound healing process.[13,14]
Several studies have demonstrated the negative role of free radicals on wound healing; reduction of persistent inflammation; and elimination of free radicals may improve healing in diabetic wounds.[15] Although advanced technologies have been developed for the treatment of diabetic wounds, outcomes have been poor. Negative pressure wound therapy (NPWT) has emerged as a treatment for complex wounds. This is a noninvasive system which creates a localized and controlled subatmospheric pressure environment. Wound healing by delayed primary or secondary intention is promoted by through the creation of a moist wound environment which prepares the wound bed for closure, reduces edema, and promotes the formation and perfusion of granulation tissue.[16,17] The clinical evidence supporting the use of continuous NPWT on diabetic wounds has been based largely on clinician perception, case series, small cohort studies, and weakly powered randomized trials that constitute a substantial number of publications but an overall low amount of evidence. Evidence is lacking on the use of an intermittent negative pressure dressing which would be more economical and clinically acceptable [17] than the use of continuous NPWT.
The present study evaluates the role of intermittent negative pressure using limited access dressing (LAD) (a cycle of 30 min of suction and 3.5 h of rest) on diabetic wounds by measuring and comparing the levels of hydroxyproline, total protein, NO, antioxidants (GSH) , and an oxidative biomarker malondialdehyde (MDA) in the granulation tissue of type-2 diabetes Mellitus ulcer patients.
Methods
Ethical approval and informed consent
This prospective randomized controlled trial study was carried out in the Department of Plastic Surgery, Kasturba Hospital, Manipal. The Institutional Ethics Committee reviewed and approved the study protocol. Written informed consent was obtained from all patients or their next of kin prior to inclusion in the study.
Study design
Patients were diagnosed on the basis of history, physical examination, and biochemical investigation. Seventy-five patients more than 40 years of age (mean age: 56 years) suffering from chronic diabetic wounds with insulin-controlled diabetes were enrolled in the study. After evaluation for inclusion (diabetic leg ulcers) and exclusion criteria (collagen disorders, leprosy, pregnancy, cirrhosis, and HIV positive status), 55 patients were randomized of whom 27 were assigned to the LAD group and 28 were assigned to the conventional dressing group. Biopsies were taken from both groups on day 0 of the study. The LAD group patients were treated with intermittent negative pressure and a moist wound environment, and wounds were washed daily with a solution of povidone-iodine. Conventional dressing group patients were dressed daily with 5% povidone-iodine solution soaked gauze. On day 10, granulation tissue biopsies were taken from both groups and subjected to biochemical study by an investigator blinded to the clinical data.
Randomization
Patients were randomized by generating tables of random numbers through www.random.org. Numbers were assigned to a treatment group and sealed in opaque envelopes containing labeled paper with the treatment and the patient’s ID.
Chemicals
All chemical used were of analytical grade. Standard L-hydroxyproline, bovine serum albumin (BSA), standard glutathione, 1-chloro-2,4-dinitrobenzene, and cumene H2O2 were purchased from Sigma-Aldrich (St. Louis, MO, USA). Thiobarbituric acid (TBA), trichloroacetic acid (TCA), 1,1,3,3-tetramethoxypropane N-ethylmaleimide (NEM), and orthophosphoric acid were purchased from S.D. Fine -Chemicals Ltd. (Boisar, India).
Tissue preparation for assays
Tissue preparation for hydroxyproline estimation
The granulation tissue specimens were dried at 60℃ for 24 h and then were weighed and kept in glass-stoppered test tubes. 6N HCl was added to each tube for a total of 40 mg of dried granulation tissue per ml of acid. The tubes were kept in boiling water bath for a total of 24 h (12 h each for 2 days) for hydrolysis. The hydrolysate was then cooled and the excess acid was neutralised by 10N sodium hydroxide (NaOH) using phenolphthalein/methyl red as an indicator. The volume of neutral hydrolysate was diluted with distilled water to a concentration of 20 mg/mL of dried granulation tissue in the final hydrolysate. The hydrolysate was used to estimate the level of hydroxyproline.[18]
Tissue preparation for estimation of antioxidants and oxidative biomarkers
The wet weight of the granulation tissue samples was noted and homogenized by a Rotex homogenizer in ice-cold 0.2 M phosphate buffer (pH 7.4). Homogenates were centrifuged at 15,000 rpm for 30 min in a cooling centrifuge and the supernatant was then used to determine total protein, GSH and an oxidative biomarker (MDA).
For the MMP-2 assay, the granulation tissue was rinsed in ice-old phosphate buffer saline (PBS) (0.1mol/L, pH 7.0-7.2) to remove all traces of excess blood and weighed prior to homogenization. The tissues were minced into small pieces and homogenized on ice in 5 mL of PBS. The resulting suspension was sonicated with an ultrasonic cell disrupter or subjected to two freeze-thaw cycles to further break the cell membranes. The homogenates were then centrifuged for 5 min at 50 g, removing the supernatant and aliquot store at no more than -80℃.
For the NO assay, the tissue was homogenized in an isotonic solution of PBS containing 10mM of NEM and 2.5 mmol ethylenediaminetetraacetic acid (EDTA). The addition of NEM/EDTA blocks the SH-groups while inhibiting the transition of metal-catalyzed trans-nitrosation reactions, preventing artificial nitrosation and thiolate- and ascorbate-mediated degradation of endogenous Nitrosothiols and nitrite (NO2−). The protein concentration was determined according to Lowry et al.[19] using purified bovine serum albumin as the standard.
Estimation of biochemical parameters
Hydroxyproline
The dried tissue was added to 10 mL of 6N HCl and stored at 110℃ for 24 h. The neutralized acid hydrolysate of the dry tissue was used to determine the level of hydroxyproline. The reaction mixture contains 0.05 M copper sulphate, 2.5N NaOH, 6% H2O2, 3N sulphuric acid, and 5% p-dimethylaminobenzaldehyde using L-hydroxyproline as the standard. Absorbance was measured at 540 nm and expressed as µg/mg of dry tissue weight.[18]
Total protein
The total protein content of the wound tissue homogenate was determined according to the method of Lowry et al.[19] Absorbance was measured at 540 nm and expressed in mg/g of tissue. Standards were treated similarly using BSA at concentrations of 0, 20, 40, 60, 80 and 100 µg/mL in 0.1 M phosphate buffer at pH 7.4.
Matrix metalloproteinase-2 and -9
A total of 100 mg of tissue was homogenized in 1 mL of ice-cold lysis buffer. Subsequently, homogenates were centrifuged at 3,000 g for 5 min at 4℃, and supernatants were stored at -80℃ until use. MMP-2 and MMP-9 were measured using prefabricated ELISA kits, according to manufacturer protocol (R and D Systems, Uscn Life Science Inc. USA). Plates were read at 450 nm and 540 nm and concentrations were calculated using a 4-point standard curve and expressed as ng/mg of protein.[20]
Nitric oxide estimation
The level of NO was estimated as NO2-, an NO metabolite, in tissue samples. NO is a highly reactive free radical gas which is a ready oxidizer and remains stored in tissues as nitrates (NO3-) or nitrite (NO2-). Thus, NO concentration can be estimated by measuring concentrations of NO3- and NO2- in combination. The simplest technique is to monitor the reduction of NO3- to NO2- by nitrate reductase or a metallic catalyst, followed by the calorimetric Griess Reaction to measure NO2- levels (nitrite levels).
Sample NO2- levels were estimated by colorimetric assay which is based on the Griess reaction. Using a two-step diazotization reaction, acidified nitrite produces a nitrosating agent which reacts with sulfa nitric acid to produce the diazonium ion. This ion is then coupled to N-(1-naphthyl) ethylenediamine dihydrocholoride to form a red azo derivative which is measured at 550 nm using a spectrophotometer. The concentrations of nitrite are calculated from a standard curve established with serial dilutions of sodium NO2-.[21]
Glutathione estimation
The reaction mixture was prepared by adding 80 μL of tissue supernatant to 0.9 mL of 0.2 mol/L sodium phosphate buffer of pH 7.00 and 20 μL of 10 mmol/L 5.5'-dithiobis (2-nitrobenzoic acid) (DTNB) solution. The yellow-colored substance formed by the reaction of GSH and DTNB was measured at 412 nm. The results were expressed as GSH µmol/mg protein.[22]
Malondialdhyde
The MDA levels of wound tissue homogenate were measured according to the method of Ohkawa et al.[23] To 0.1 mL of test sample, 1 mL of 10% TCA and 1 mL of 0.67% TBA were added and then heated in a boiling water bath at 100℃ for 30 min. The mixture was cooled under tap water and centrifuged at 12,000 rpm for 10 min. Clear supernatant was determined by the absorbance at 535 nm and expressed as nmol/mg protein.
Statistical analysis
Statistical analysis for biochemical parameters was performed by Student’s t-test and data were expressed as mean ± standard deviation (SD). P < 0.05 was considered to be significant. When appropriate, statistical uncertainty was expressed by 95% confidence levels.
Results
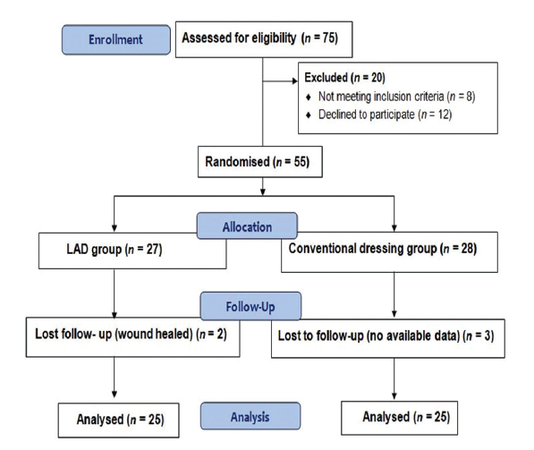
A total of 75 patients were enrolled of which 55 patients were randomized, with 27 patients assigned to the LAD group and 28 patients assigned to the conventional group [Figure 1]. Five participants were lost to follow-up (two in the LAD and three in the conventional group) before the biopsies could be taken. The results of hydroxyproline, total protein, MMP-2 and MMP-9, GSH, NO, and MDA levels in diabetic ulcers are shown [Table 1].
Results of hydroxyproline, total protein, MMP-2 and MMP-9, NO, GSH, and MDA levels in granulation tissue of diabetic ulcer in LAD group and conventional dressing group
| Parameters | Mean ± SD | P | |||||
|---|---|---|---|---|---|---|---|
| LAD group | Conventional dressing group | ||||||
| Day 0 | Day 10 | Day (0-10) | Day 0 | Day 10 | Day (0-10) | ||
| Hydroxyproline (μg/mg of dry weight of tissue) | 46.9 ± 16.0 | 100 ± 6.6 | 55.2 ± 25.1 | 51.5 ± 10.9 | 80.7 ± 15.6 | 29.2 ± 13.5 | 0.000 |
| Total protein (mg/g of wet weight tissue) | 11.7 ± 3.2 | 24.3 ± 5.9 | 12.8 ± 6.5 | 12.0 ± 2.6 | 20.6 ± 4.8 | 8.34 ± 3.2 | 0.011 |
| MMP-2 (ng/mg tissue protein) | 0.95 ± 0.20 | 0.48 ± 0.42 | 0.47 ± 0.33 | 0.97 ± 0.52 | 0.35 ± 0.49 | 0.62 ± 0.30 | 0.041 |
| MMP-9 (ng/mg tissue protein) | 0.87 ± 0.54 | 0.55 ± 0.25 | 0.32 ± 0.20 | 0.93 ± 0.22 | 0.40 ± 0.32 | 0.53 ± 0.39 | 0.037 |
| NO (nmol/mg protein) | 2.0 ± 1.42 | 3.13 ± 1.3 | 1.13 ± 0.52 | 2.74 ± 1.4 | 3.4 ± 1.44 | 0.66 ± 0.43 | 0.019 |
| GSH (μmol/mg protein) | 16.1 ± 4.3 | 23.1 ± 3.3 | 7.0 ± 2.4 | 14.9 ± 3.5 | 21.5 ± 3.79 | 6.6 ± 2.2 | 0.044 |
| MDA (nmol/mg protein) | 19.3 ± 3.8 | 12.5 ± 4.8 | 6.8 ± 2.3 | 19.9 ± 4.5 | 9.5 ± 4.5 | 10.4 ± 3.4 | 0.024 |
Hydroxyproline
After 10 days of treatment, the LAD group had significantly higher hydroxyproline level (mean ± SD = 55.2 ± 25.1 µg/mg dry tissue weight) than the conventional group (29.2 ± 13.5 µg/mg dry tissue weight), P = 0.000.
Total protein
After 10 days of treatment, the LAD group had significantly higher total protein levels (mean ± SD = 12.8 ± 6.5 mg/g wet tissue weight) than the conventional group (8.34 ± 3.2 mg/g wet tissue weight), P = 0.011.
Matrix metalloproteinases-2 and matrix metalloproteinases-9
After 10 days of treatment, MMP-2 (0.47 ± 0.33 ng/mg tissue protein; P = 0.041) and MMP-9 levels (0.32 ± 0.20 ng/mg tissue protein; P = 0.037) in LAD group were significantly lower than the conventional group MMP-2 (0.62 ± 0.30 ng/mg tissue protein) and MMP-9 (0.53 ± 0.39 ng/mg tissue protein.
Nitric oxide
After 10 days of treatment, the LAD group had a significantly high total protein level (mean ± SD = 1.13 ± 0.52 nmol/mg protein) than the conventional group (0.66 ± 0.43 nmol/mg protein), P = 0.019.
Reduced glutathione
After 10 days of treatment, antioxidant (reduced GSH) levels in the LAD group were significantly higher (mean ± SD = 7.0 ± 2.4 µmol/mg protein) as compared to those in the conventional dressing group (6.6 ± 2.2 µmol/mg protein), P = 0.044.
Malondialdehyde
After 10 days of treatment, MDA levels were significantly higher in the conventional dressing group (mean ± SD = 10.4 ± 3.4 nmol/mg protein) as compared to the LAD group (6.8 ± 2.3 nmol/mg protein), P = 0.024.
Discussion
Diabetes is a multisystem disorder which induces physiological changes in tissues and cells that impair the normal healing process. Diabetic wounds can have prolonged periods in the inflammatory phase of healing, with a continuous influx of neutrophils that release cytotoxic enzymes, free radicals and inflammatory mediators, causing extensive collateral damage to the surrounding tissue.[24-26]
In the treatment of diabetic ulcers, the NPWT wound dressing provides faster wound resolution as compared to gauze dressings. NPWT may be beneficial in the treatment of nonhealing diabetic wounds of the lower extremity.[27] The intermittent negative pressure regimen used in LAD has been shown to be an economical and effective alternative in treating traumatic wounds.[28,29]
Evidence shows that increased oxidative stress causes delayed wound healing in diabetics with altered antioxidant enzyme activities.[30] Various studies[31,32] have shown a significant reduction in collagen content in the granulation tissue of diabetic animals. The authors of these studies have proposed that the decreased collagen levels seen in diabetic wounds are likely in response to an altered extracellular environment (i.e. hyperglycemia, persistent inflammation, excess H2O2 and free radical production and low levels of antioxidants). Collagen is the major component of extracellular tissue which provides support and strength. It is measured by monitoring the concentration of hydroxyproline that is synthesized by fibroblasts[33] and is vulnerable to the effects of free radicals. In the present study after 10 days of therapy, the mean level of hydroxyproline (± SD) was significantly higher in the LAD group as compared to that of the conventional dressing group (LAD vs. conventional = 55.2 ± 25.1 vs. 29.2 ± 13.5 µg/mg of dry weight of tissue; P = 0.000). The concentration of protein found in the wound’s granulation tissue was higher in the LAD group (12.8 ± 6.5 mg/g wet tissue weight) than in the conventional group (8.34 ± 3.2 mg/g wet tissue weight), P = 0.011.
In a study by Lobmann et al.[34] higher concentrations of the MMP-2 and MMP-9, in conjunction with lower concentrations of TIMP-2, were found in tissue biopsies of diabetic wounds as compared to healthy controls.[34] Another study by Liu et al.[35] showed that high MMP-9 levels were indicative of poor wound healing in diabetics In the present study significant decreases in the concentrations of MMP-2 (0.47 ± 0.33 ng/mg tissue protein; P = 0.041) and MMP-9 (0.32 ± 0.20 ng/mg tissue protein; P = 0.037) were observed in the LAD group vs. the conventional group after 10 days of treatment.
Normal physiological amounts of NO act as a mediator for moderate vasodilatation and are required to increase blood flow and vascular permeability. Endothelial dysfunction and decreased NO production are observed in diabetic patients with a consequent delay in wound healing due to decreased tissue perfusion.[36] After 10 days of therapy the mean level of NO ( ± SD) was significantly higher in the LAD group as compared to that in the conventional dressing group (LAD vs. conventional = 1.13 ± 0.52 vs. 0.66 ± 0.43 nmol/mg protein; P = 0.019).
Decreased levels of the enzymatic antioxidants, GPx, CAT, and GSH may contribute to diminished defense mechanisms against free radical overload in diabetic wounds.[37] Antioxidants hasten the process of wound healing by destroying free radicals.[38] In the present study, after 10 days of treatment the levels of antioxidants in granulation tissue were significantly higher in the LAD group as compared to the conventional dressing group (LAD vs. conventional = 7.0 ± 2.4 vs. 6.6 ± 2.2 µmole/mg protein; P = 0.044). Lipid peroxidation is an important pathophysiological event in diabetes.[39] It is well known that MDA from lipid peroxidation reacts with DNA bases and induces mutagenic lesions.[40] In the present study after 10 days of therapy, the mean level of MDA (± SD) was significantly less in the LAD group than in the conventional dressing group (LAD vs. conventional = 6.8 ± 2.3 vs. 10.4 ± 3.4 nmol/mg protein; P = 0.024).
In conclusion, LAD (intermittent NPWT and moist wound dressing) exerts a beneficial effect on diabetic wound healing as seen by a significant increase in the levels hydroxyproline, total protein, NO, antioxidants, a decrease in MMP-2 and MMP-9 and oxidative biomarkers (MDA) as compared to conventional dressings.
The use of intermittent NPWT using LAD may be an effective alternative therapy used to achieve faster granulation of the wound bed in diabetic ulcers.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
2. Eming SA, Krieg T, Davidson JM. Inflammation in wound repair: molecular and cellular mechanisms. J Invest Dermatol 2007;127:514-25.
3. Khodr B, Khalil Z. Modulation of inflammation by reactive oxygen species: implications for aging and tissue repair. Free Radic Biol Med 2001;30:1-8.
4. Hallberg CK, Trocme SD, Ansari NH. Acceleration of corneal wound healing in diabetic rats by the antioxidant Trolox. Res Comm Mol Pathol Pharmacol 1996;93:3-12.
5. McDaniel DH, Ash K, Lord J, Newman J, Zukowski M. Accelerated laser resurfacing wound healing using a triad of topical antioxidants. Dermatol Surg 1998;24:661-4.
6. Madlener M, Parks WC, Werner S. Matrix metalloproteinases and their physiological inhibitors (TIMPs) are differentially expressed during excisional skin wound repair. Exp Cell Res 1998;242:201-10.
7. Kakkar R, Kalra J, Mantha SV, Prasad K. Lipid peroxidation and activity of antioxidant enzymes in diabetic rats. Mol Cell Biochem 1995;151:113-9.
8. Wiseman H, Halliwell B. Damage to DNA by reactive oxygen and nitrogen species: role in inflammatory disease and progression to cancer. Biochem J 1996;313:17-29.
9. Sivitz WI, Yorek MA. Mitochondrial dysfunction in Diabetes: from molecular mechanisms to functional significance and therapeutic opportunities. Antioxid Redox Signal 2010;12:537-77.
10. Rolo AP, Palmeira CM. Diabetes and mitochondrial function: role of hyperglycemia and oxidative stress. Toxicol Appl Pharmacol 2006;212:167-78.
12. Hsu WT, Tsai LY, Lin SK, Hsiao JK, Chen BH. Effects of diabetes duration and glycemic control on free radicals in children with type1 diabetes mellitus. Ann Clin Lab Sci 2006;36:174-8.
13. Flora SJ. Structural, chemical and biological aspects of antioxidants for strategies against metal and metalloid exposure. Oxid Med Cell Longev 2009;2:191-206.
14. Valko M, Leibfritz D, Moncol J, Cronin MT, Mazur M, Telser J. Free radicals and antioxidants in normal physiological functions and human disease. Int J Biochem Cell Biol 2007;39:44-84.
15. Honnegowda TM, Kumar P, Udupa P, Rao P, Bhandary S, Mahato KK, Sharan A, Mayya SS. Effect of limited access dressing on hydroxyproline and enzymatic antioxidant status in nonhealing chronic ulcers. Indian J Plast Surg 2014;47:216-20.
16. Polack G, McCray V, Tyner T, Kane S, Vu K, Yamaguchi K Jr, Merriman J, Ishimoto M, Hasson A, Sian K, Yamaguchi KT. Accelerated wound closure in a diabetic mouse model after exposure to phenanthrenequinone. Ann Plast Surg 2013;70:720-5.
17. Blume PA, Walters J, Payne W, Ayala J, Lantis J. Comparison of negative pressure wound therapy using vacuum-assisted closure with advanced moist wound therapy in the treatment of diabetic foot ulcers: a multicenter randomized controlled trial. Diabetes Care 2008;31:631-66.
18. Neuman RE, Logan MA. The determination of collagen and elastin in tissues. J Biol Chem 1950;86:549-56.
19. Lowry OH, Rosebrough MH, Farr L, Randell RJ. Protein measurement with the Folin phenol reagent. J Biol Chem 1951;193:265-75.
20. Young PK, Grinnell F. Metalloproteinase activation cascade after burn injury: a longitudinal analysis of the human wound environment. J Invest Dermatol 1994;103:660-4.
21. Green LC, Wagner DA, Glogowski J, Skipper PL, Wishnok JS, Tannenbaum SR. Analysis of nitrate, nitrite, and [15N] nitrate in biological fluids. Anal Biochem 1982;126:131-8.
22. Beutler E, Duron O, Kefly BM. Improved method for the determination of blood glutathione. J Lab Clin Med 1963;61:882-8.
23. Ohkawa H, Ohishi N, Yagi K. Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal Biochem 1979;95:351-8.
24. Abd-El-Aleem SA, Ferguson MW, Appleton I, Kairsingh S, Jude EB, Jones K, Mc Collum CN, Ireland GW. Expression of nitric oxide synthase isoforms and arginase in normal human skin and chronic venous leg ulcers. J Pathol 2000;191:434-42.
25. Rojkind M, Domínguez-Rosales JA, Nieto N, Greenwel P. Role of hydrogen peroxide and oxidative stress in healing responses. Cell Mol Life Sci 2002;59:1872-91.
26. Moseley R, Hilton JR, Waddington RJ, Harding KG, Stephens P, Thomas DW. Comparison of oxidative stress biomarker profiles between acute and chronic wound environments. Wound Repair Regen 2004;12:419-29.
27. Joseph E, Hamori CA, Bergman S, Roaf E, Swann NF, Anastasi GW. A prospective randomized trial of vacuum-assisted closure versus standard therapy of chronic nonhealing wounds. Wounds 2000;12:60-7.
28. Kumar P. Exploiting potency of negative pressure in wound dressing using limited access dressing and suction-assisted dressing. Indian J Plast Surg 2012;45:302-15.
29. Kumar P, Sharma A. The limited access dressing for damage control in trauma patients. Wounds 2010;22:188-92.
30. Rasik AM, Shukla A. Antioxidant status in delayed healing type of wounds. Int J Exp Pathol 2000;81:257-63.
31. Tengrup I, Hallmans G, Agren MS. Granulation tissue formation and metabolism of zinc and copper in alloxan-diabetic rats. Scand J Plast Reconstr Surg Hand Surg 1988;22:41-5.
32. Spanheimer RG, Umpierrez GE, Stumpf V. Decreased collagen production in diabetic rats. Diabetes 1988;37:371-6.
33. Honnegowda TM, Kumar P, Padmanabha Udupa EG, Sharan A, Singh R, Prasad HK, Rao P. Effects of limited access dressing in chronic wounds: A biochemical and histological study. Indian J Plast Surg 2015;48:22-8.
34. Lobmann R, Ambrosch A, Schultz G, Waldmann K, Schiweck S, Lehnert H. Expression of matrix-metalloproteinases and their inhibitors in the wounds of diabetic and non-diabetic patients. Diabetologia 2002;45:1011-6.
35. Liu Y, Min D, Bolton T, Nubé V, Twigg SM, Yue DK, McLennan SV. Increased matrix metalloproteinase-9 predicts poor wound healing in diabetic foot ulcers. Diabetes Care 2009;32:117-9.
36. Boykin JV, Jr. Wound nitric oxide bioactivity: a promising diagnostic indicator for diabetic foot ulcer management. J Wound Ostomy Continence Nurs 2010;37:25-32.
37. Johansen JS, Harris AK, Rychly DJ, Ergul A. Oxidative stress and the use of antioxidants in diabetes: linking basic science to clinical practice. Cardiovasc Diabetol 2005;4:5.
38. Honório-França AC, Marins CM, Boldrini F, França EL. Evaluation of hypoglycemic activity and healing of extract from amongst bark of "Quina do Cerrado" (Strychnos pseudoquina ST. HILL). Acta Cir Bras 2008;23:504-10.
39. Ceriello A, Quatraro A, Caretta F, Varano R, Giugliano D. Evidence for a possible role of oxygen free radicals in the abnormal functional arterial vasomotion in insulin dependent diabetes. Diabete Metab 1990;16:318-22.
Cite This Article
Export citation file: BibTeX | RIS
OAE Style
Honnegowda TM, Kumar P, Prabhu K, Kumar A, Rao P, Udupa EGP, Kamath S, Souza ASD, Mahato KK. A comparative study to evaluate the effect of limited access dressing on diabetic ulcers. Plast Aesthet Res 2015;2:266-71. http://dx.doi.org/10.4103/2347-9264.165448
AMA Style
Honnegowda TM, Kumar P, Prabhu K, Kumar A, Rao P, Udupa EGP, Kamath S, Souza ASD, Mahato KK. A comparative study to evaluate the effect of limited access dressing on diabetic ulcers. Plastic and Aesthetic Research. 2015; 2: 266-71. http://dx.doi.org/10.4103/2347-9264.165448
Chicago/Turabian Style
Honnegowda, Thittamaranahalli Muguregowda, Pramod Kumar, Krishnananda Prabhu, Ashwini Kumar, Pragna Rao, E G Padmanabha Udupa, Shobha Kamath, Antony Sylvan D' Souza, Krishna Kishore Mahato. 2015. "A comparative study to evaluate the effect of limited access dressing on diabetic ulcers" Plastic and Aesthetic Research. 2: 266-71. http://dx.doi.org/10.4103/2347-9264.165448
ACS Style
Honnegowda, TM.; Kumar P.; Prabhu K.; Kumar A.; Rao P.; Udupa EGP.; Kamath S.; Souza ASD.; Mahato KK. A comparative study to evaluate the effect of limited access dressing on diabetic ulcers. Plast. Aesthet. Res. 2015, 2, 266-71. http://dx.doi.org/10.4103/2347-9264.165448
About This Article
Special Issue
Copyright
Data & Comments
Data
 Cite This Article 1 clicks
Cite This Article 1 clicks











Comments
Comments must be written in English. Spam, offensive content, impersonation, and private information will not be permitted. If any comment is reported and identified as inappropriate content by OAE staff, the comment will be removed without notice. If you have any queries or need any help, please contact us at support@oaepublish.com.